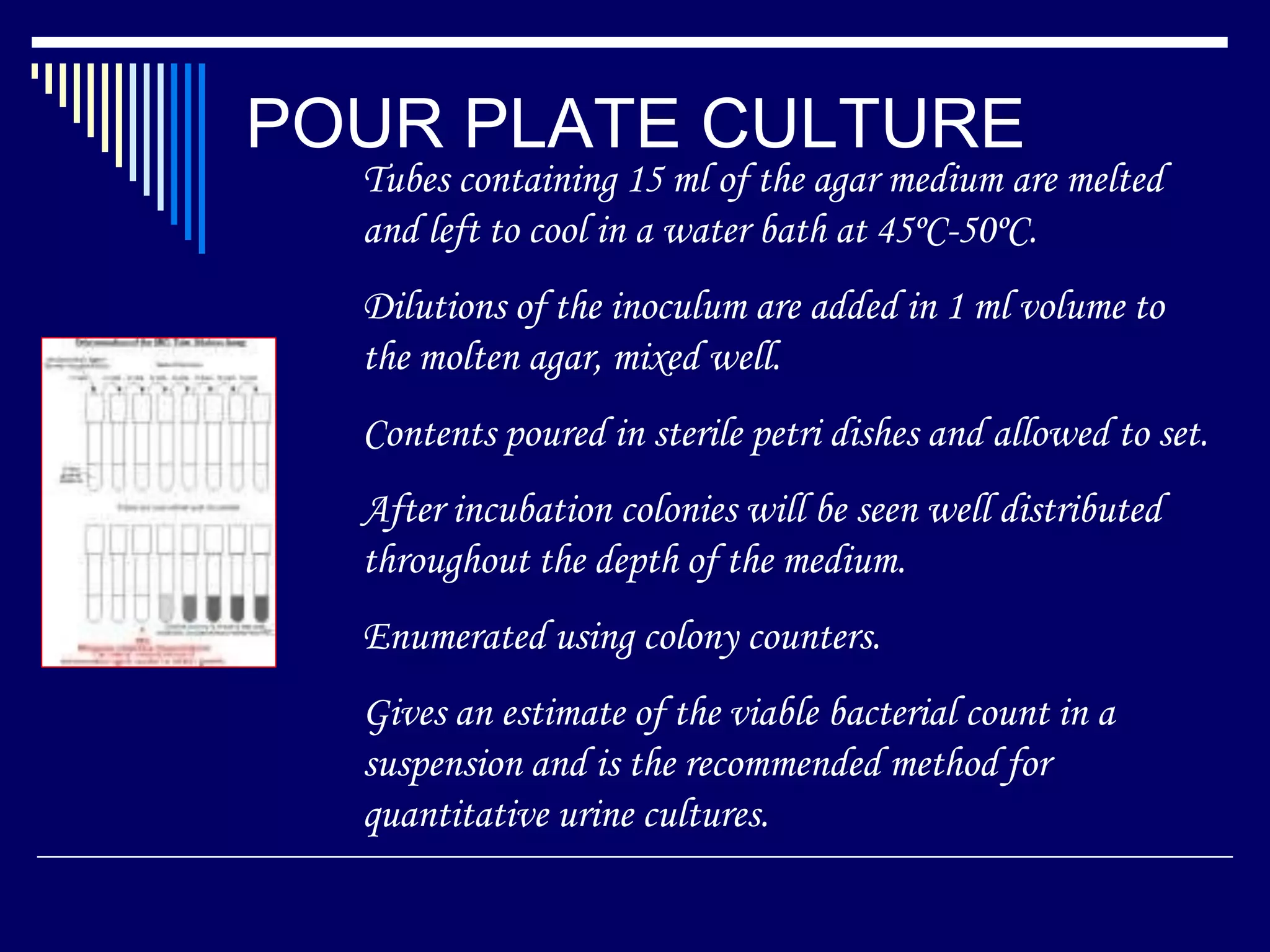
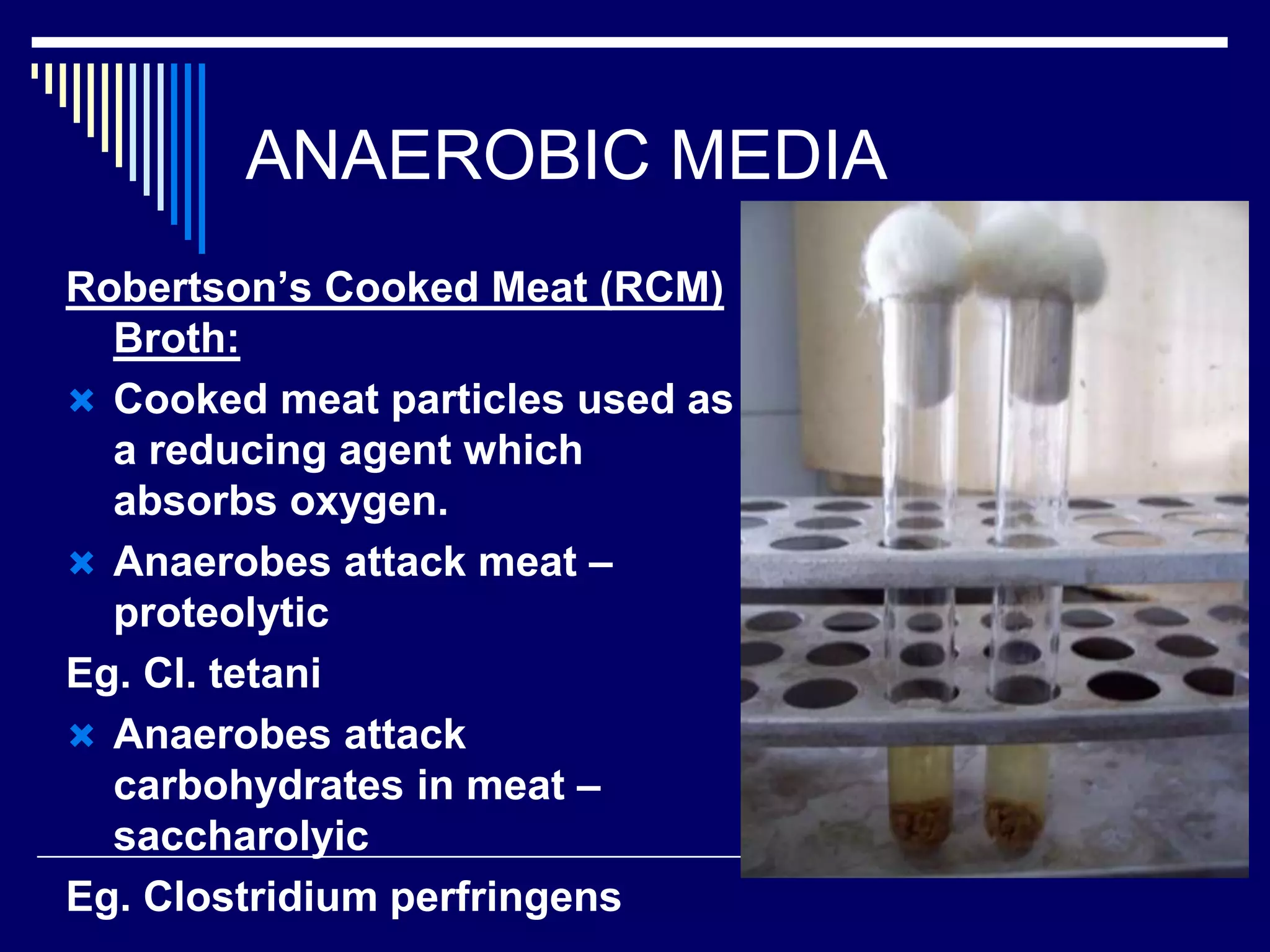
This document discusses various culture methods used to isolate and grow bacteria. It describes streak culture, lawn culture, stroke culture, stab culture, pour plate culture, and liquid culture. Streak culture is routinely used to isolate bacteria by streaking an inoculum across agar plates. Lawn culture provides uniform bacterial growth. Stroke and stab cultures are used to provide pure bacterial growth for diagnostic tests. Pour plate culture allows enumeration of bacterial counts. Liquid cultures are used for blood cultures and sterility tests. The document also discusses methods for establishing anaerobic conditions and various anaerobic media.