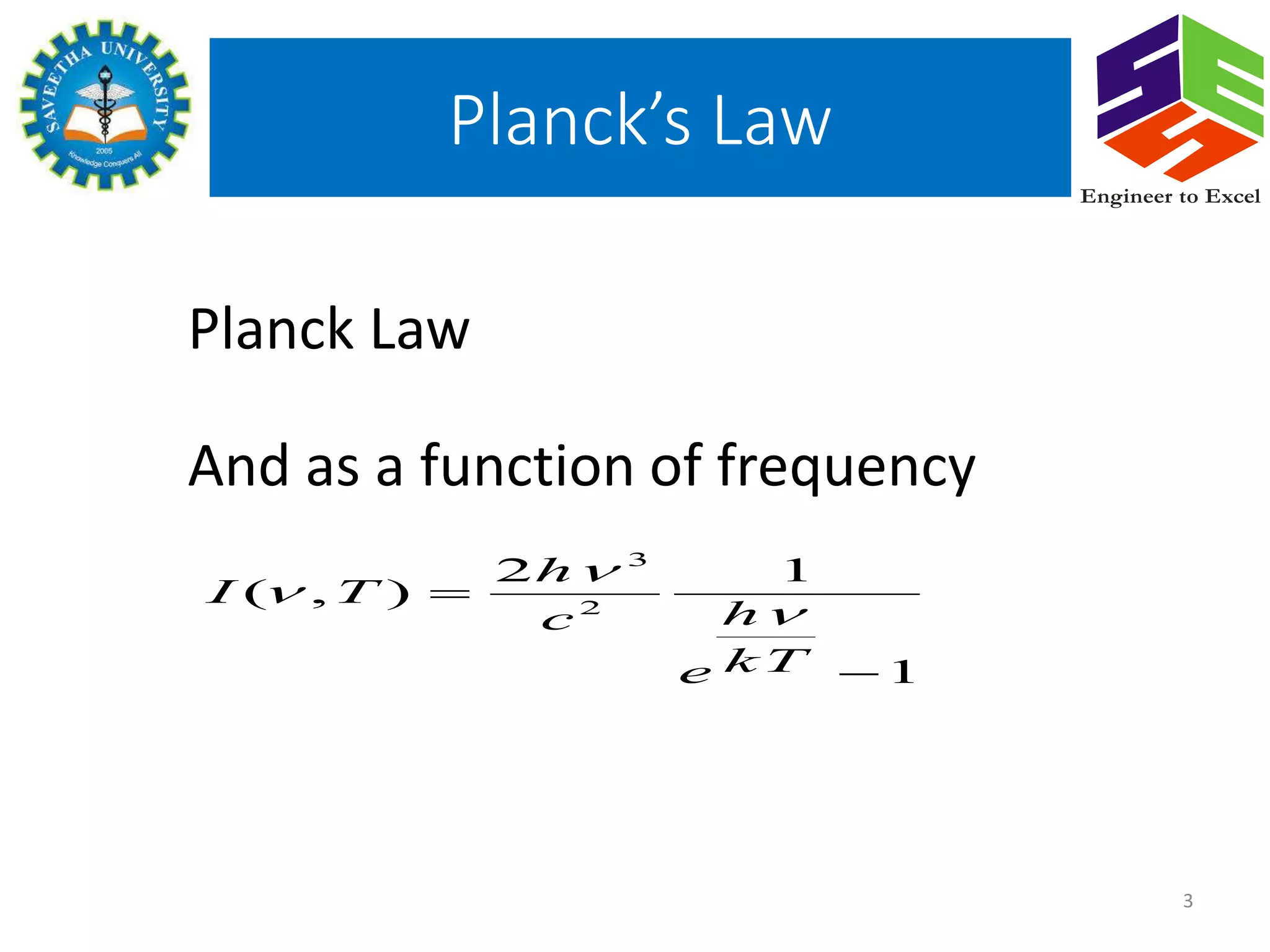
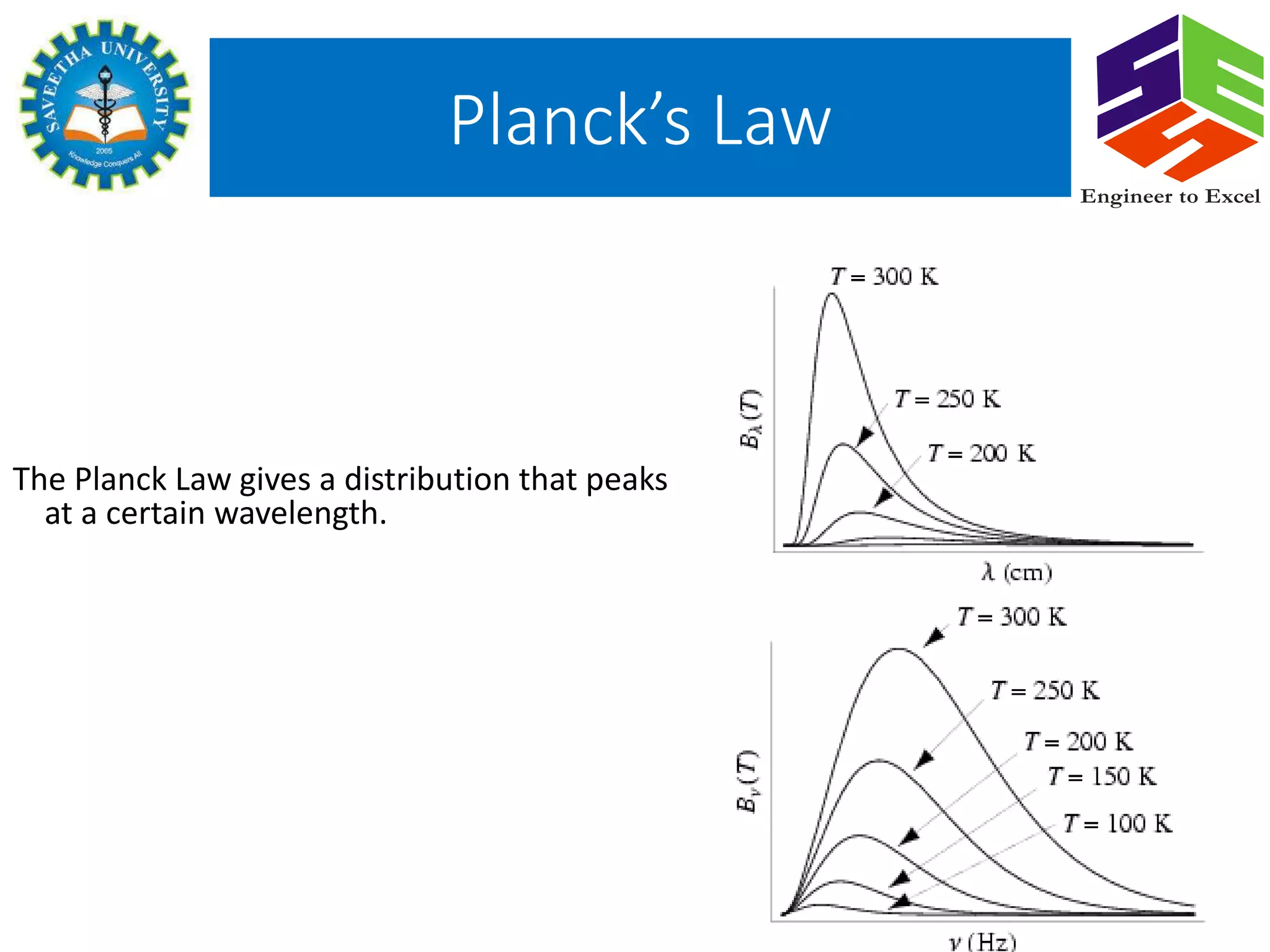
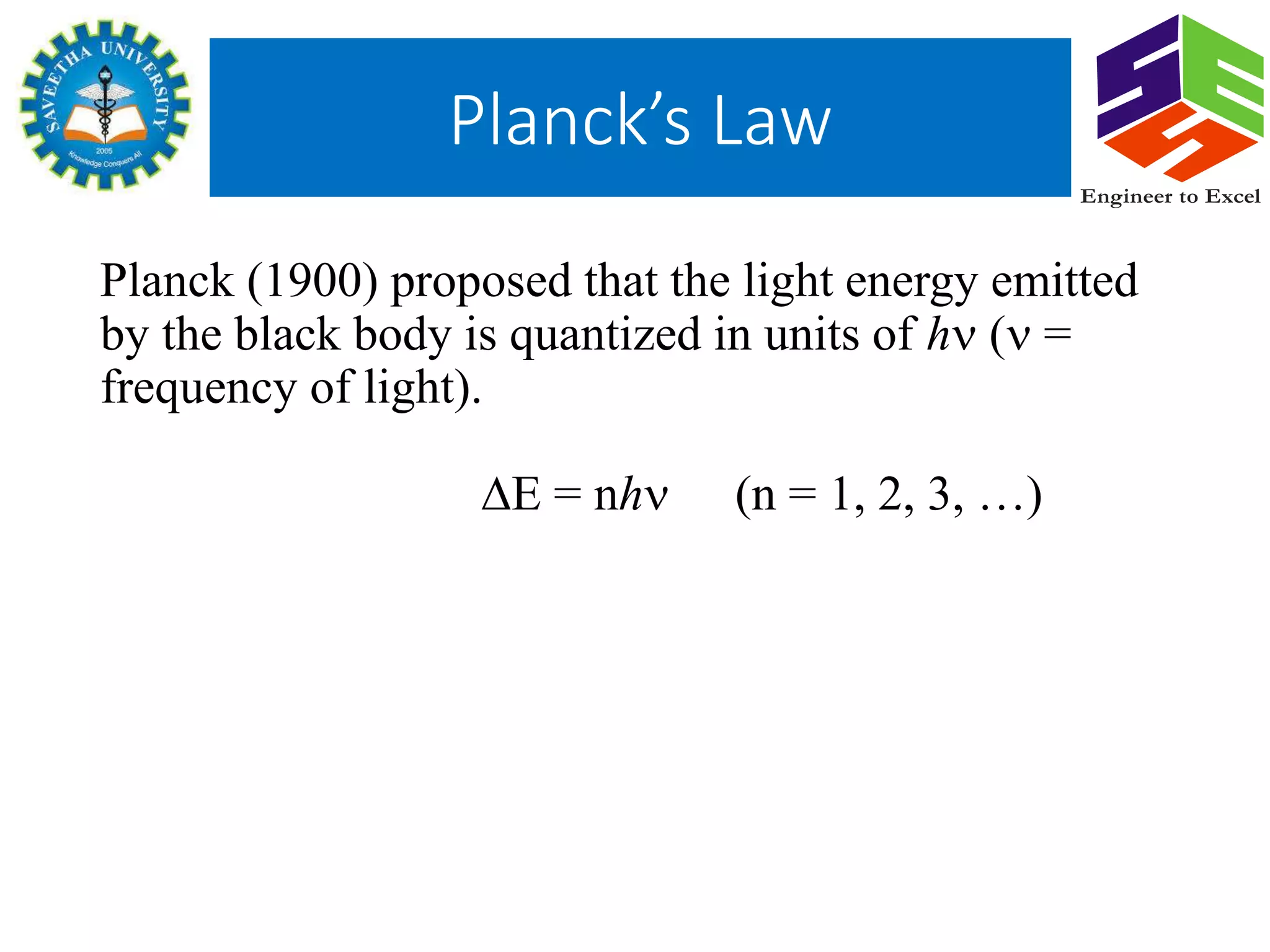
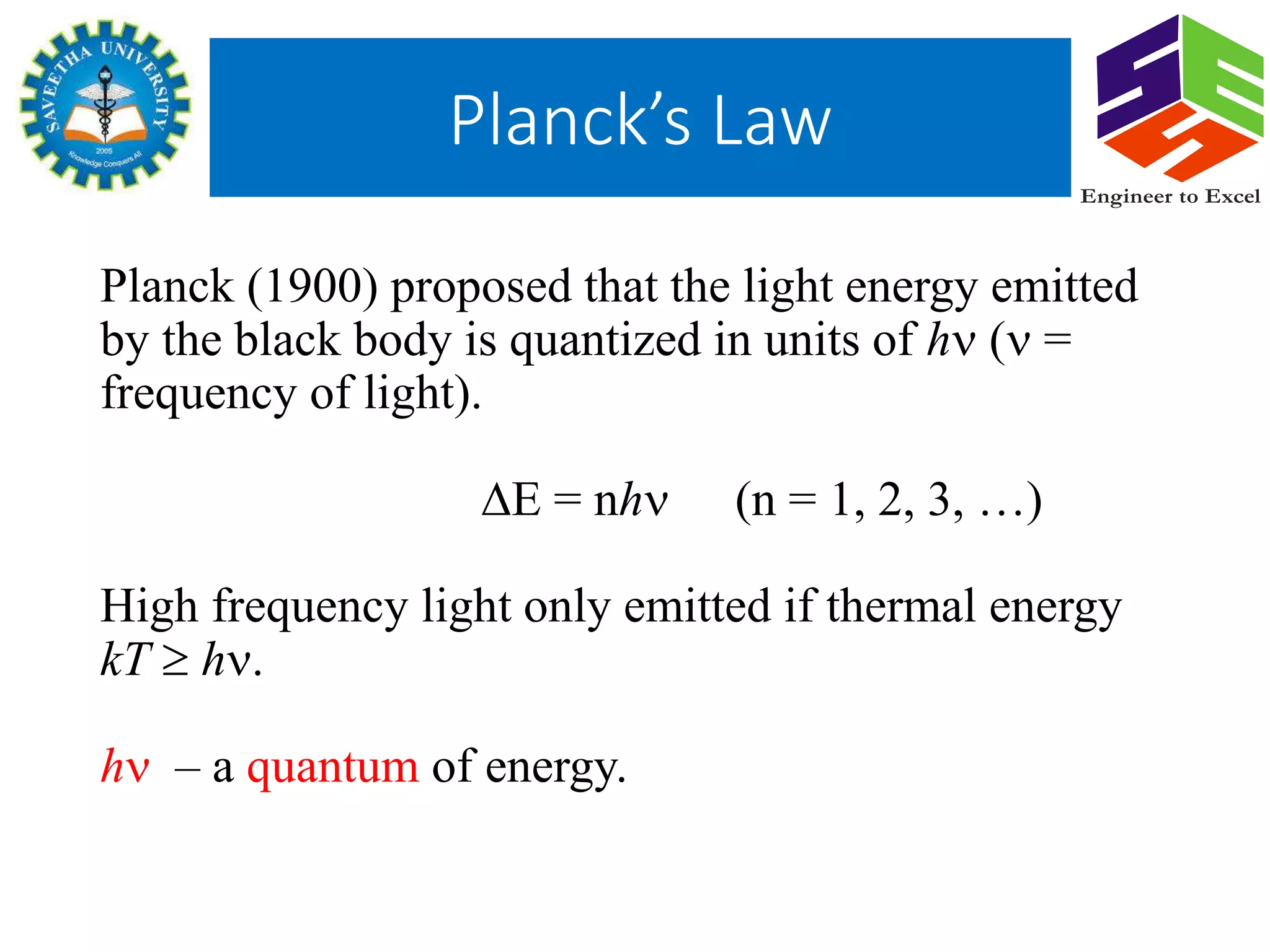
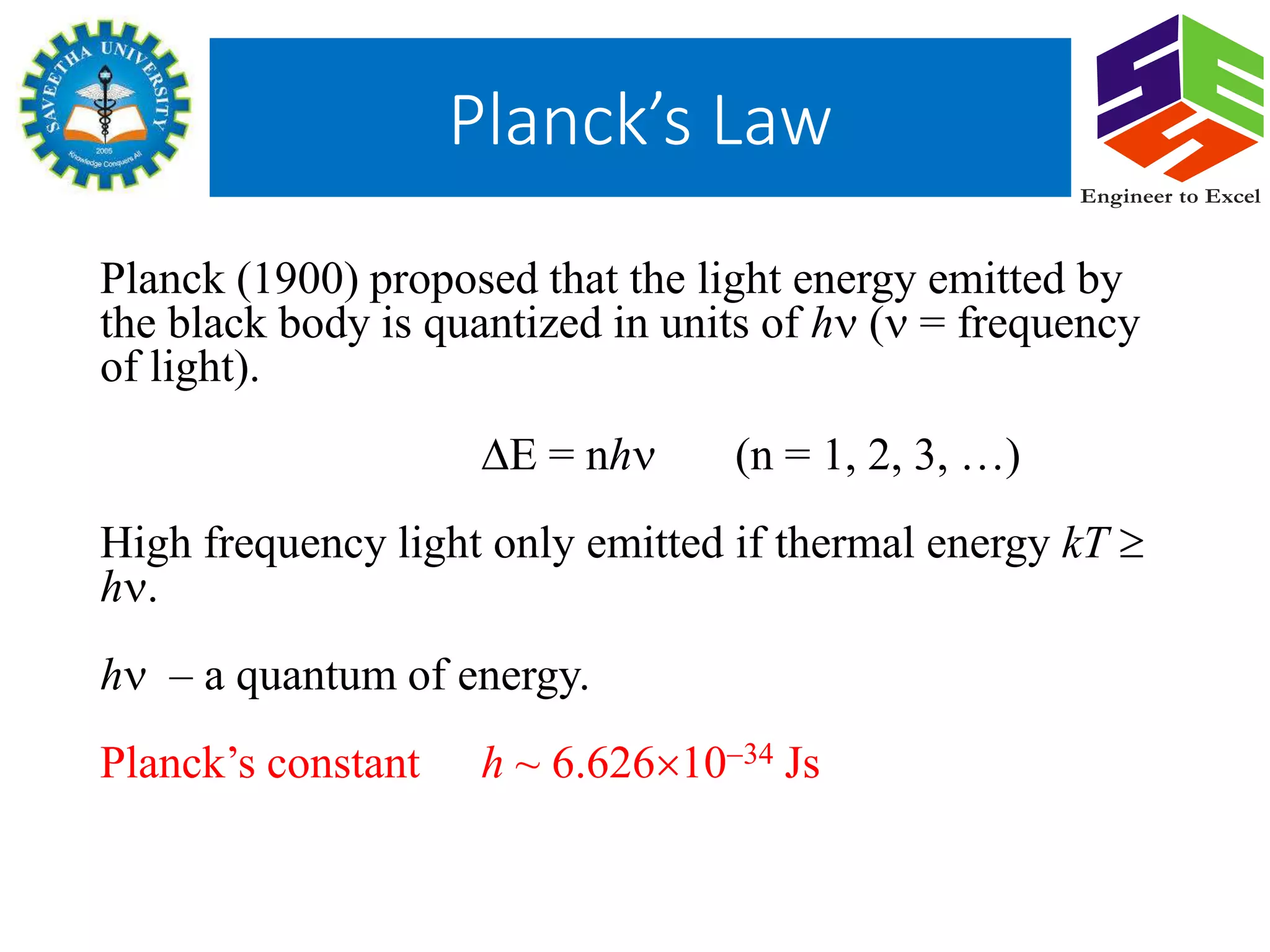
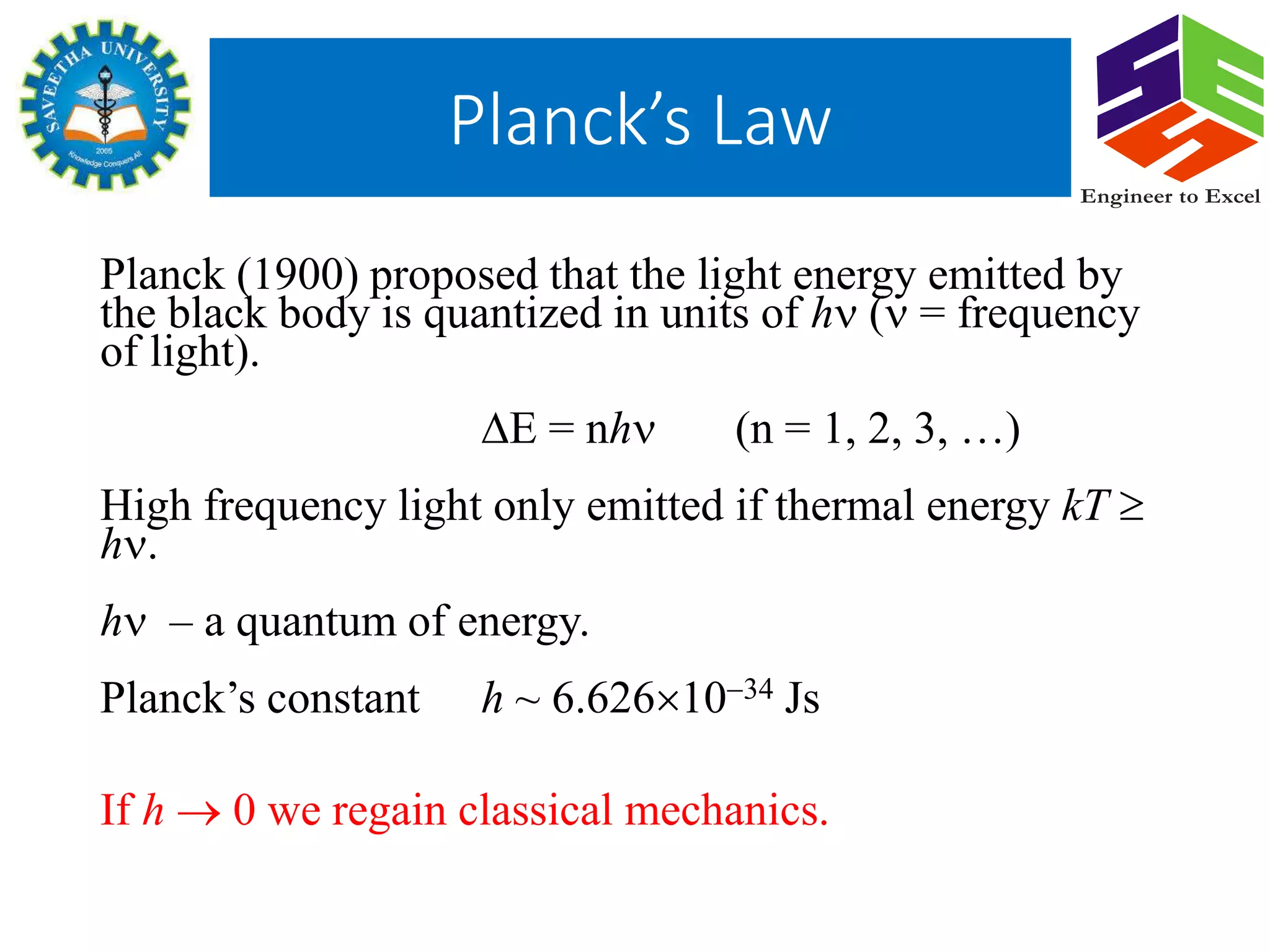
Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium based on two parameters: temperature and frequency or wavelength. It provides a distribution that peaks at a certain wavelength, with the peak shifting to shorter wavelengths at higher temperatures. Planck proposed that the light energy emitted by a black body is quantized in units of Planck's constant h multiplied by the frequency of light. This resolved the ultraviolet catastrophe and established the foundation for quantum mechanics.