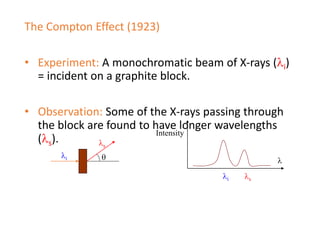
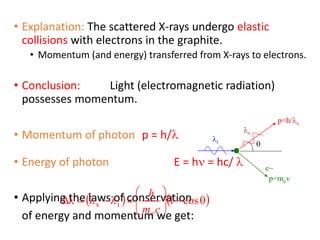
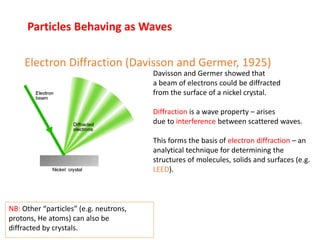
The document discusses several experiments from 1905-1925 that provided evidence that challenged classical physics and led to the development of quantum theory, including:
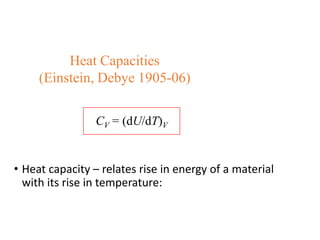
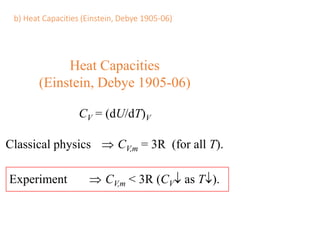
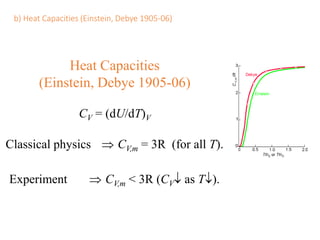
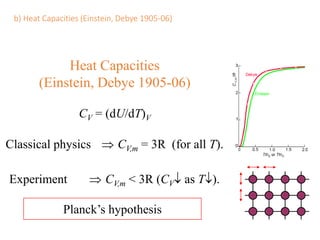
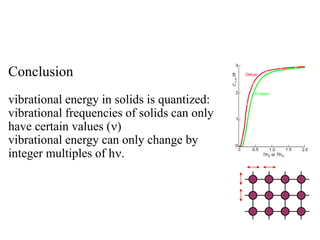
- Heat capacity experiments showing heat capacity decreases with temperature, contradicting classical physics (1905-1906)
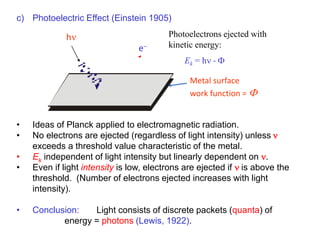
- Photoelectric effect experiments showing electrons ejected by frequency-dependent photons, not intensity (1905)
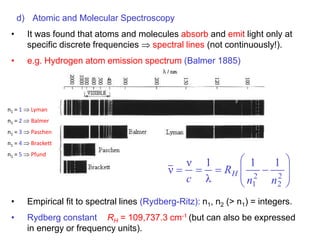
- Atomic spectroscopy experiments showing atoms absorb/emit only discrete frequencies (1885, early 1900s)