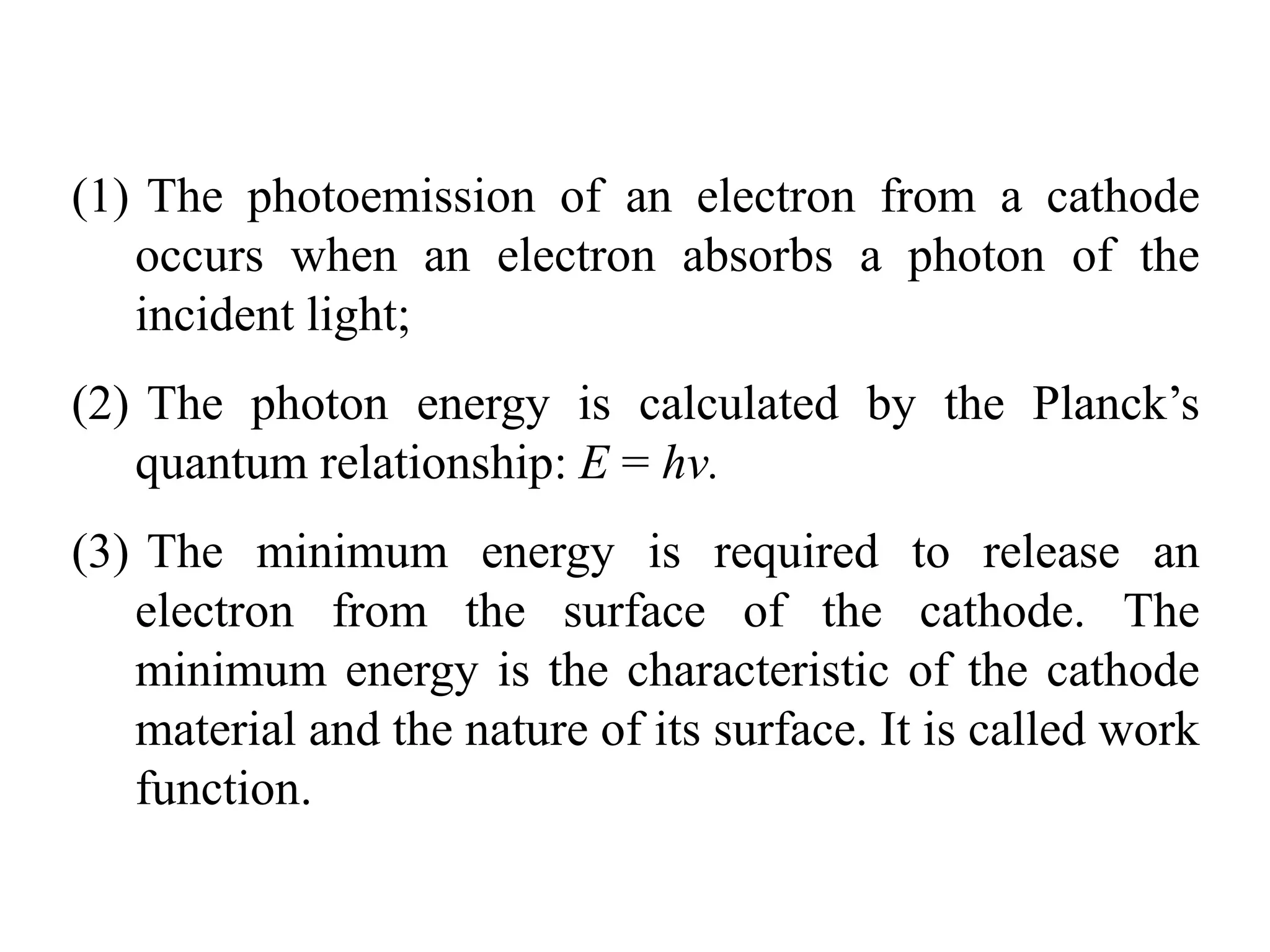
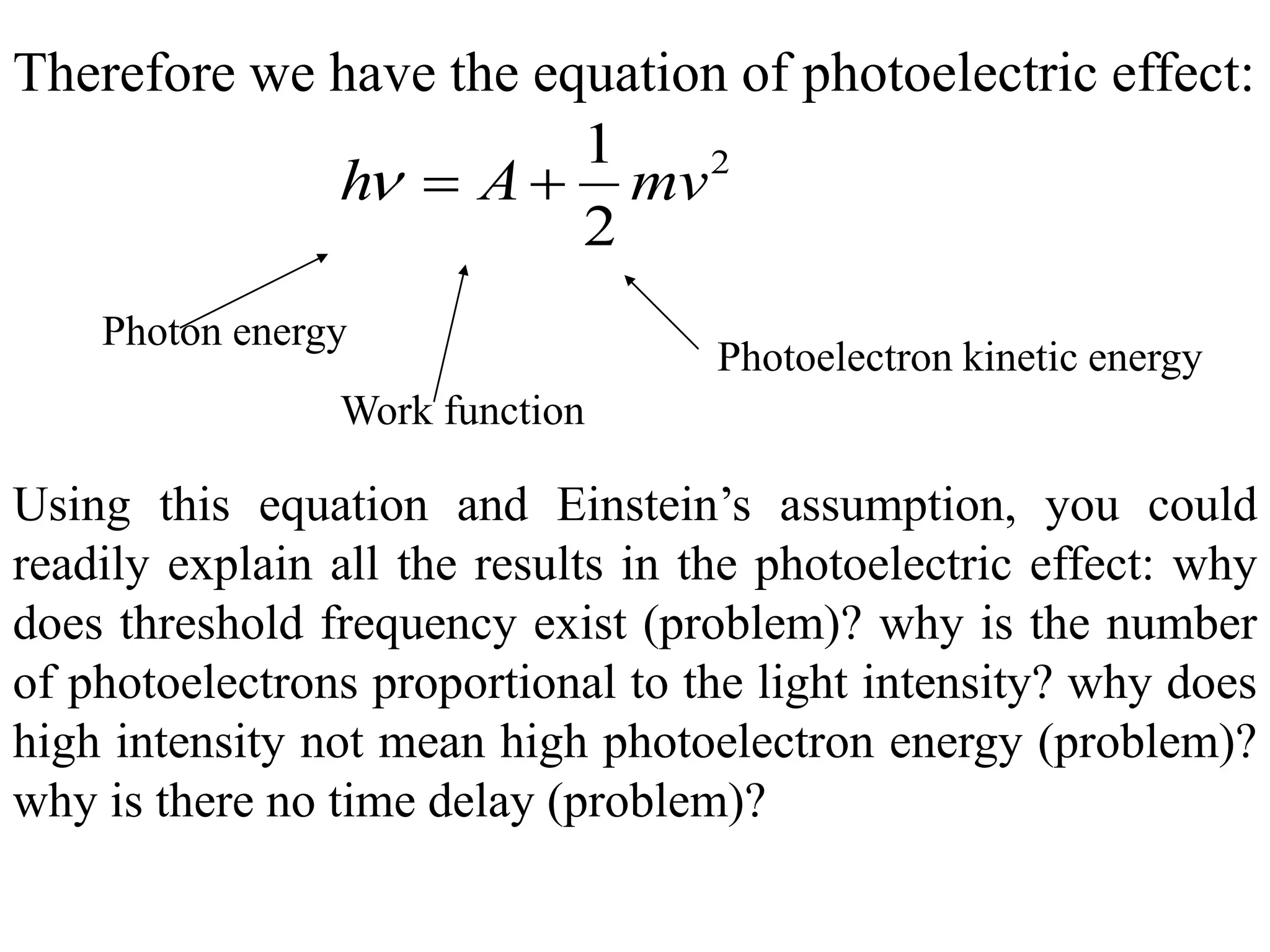
1. Classical physics describes large, everyday objects but fails to describe phenomena at the atomic scale. Quantum physics was developed in the early 20th century to address these shortcomings.
2. Quantum physics is counterintuitive, but it is the most successful physical theory ever developed. It underlies our understanding of atoms, molecules, and subatomic particles.
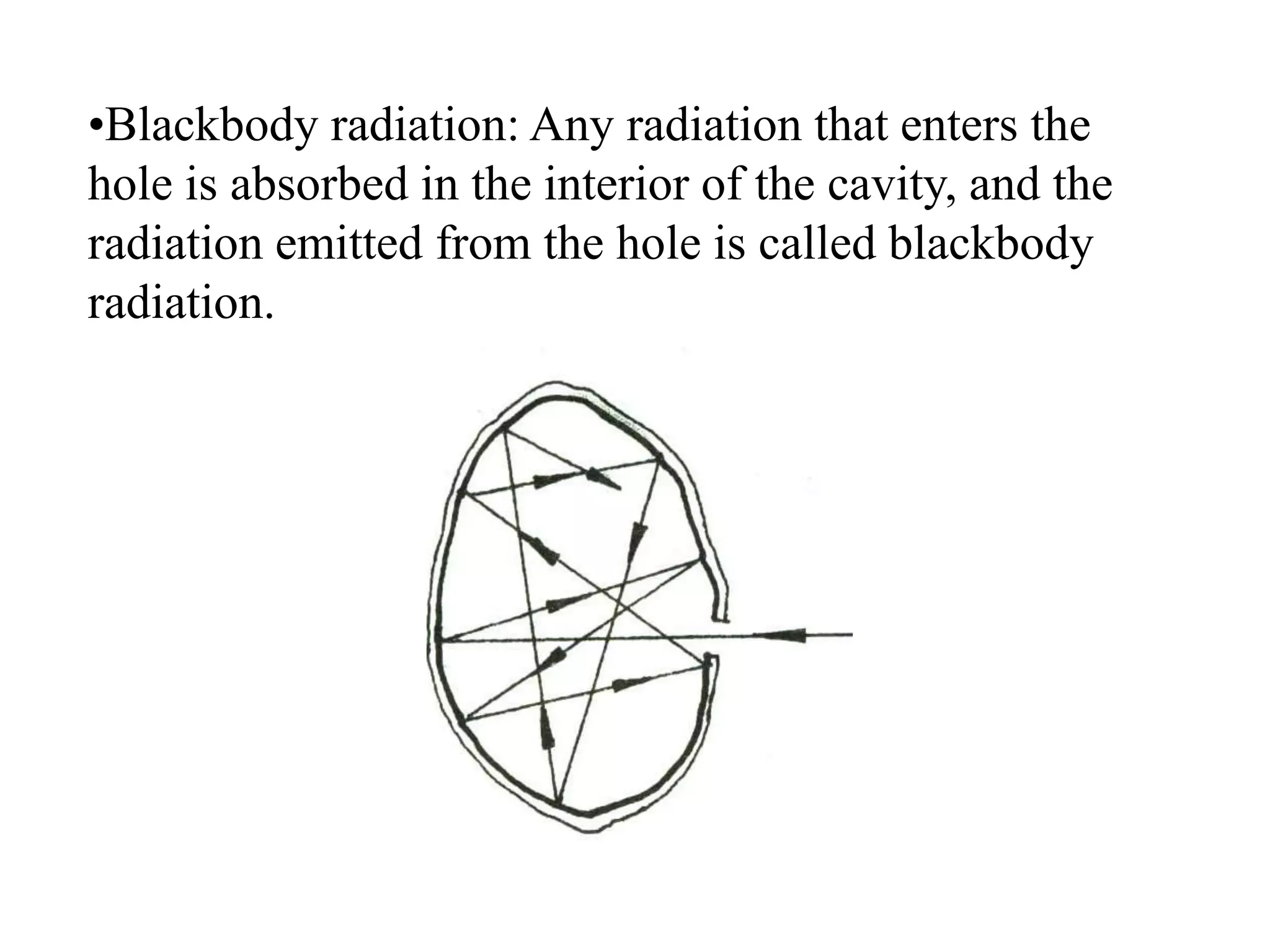
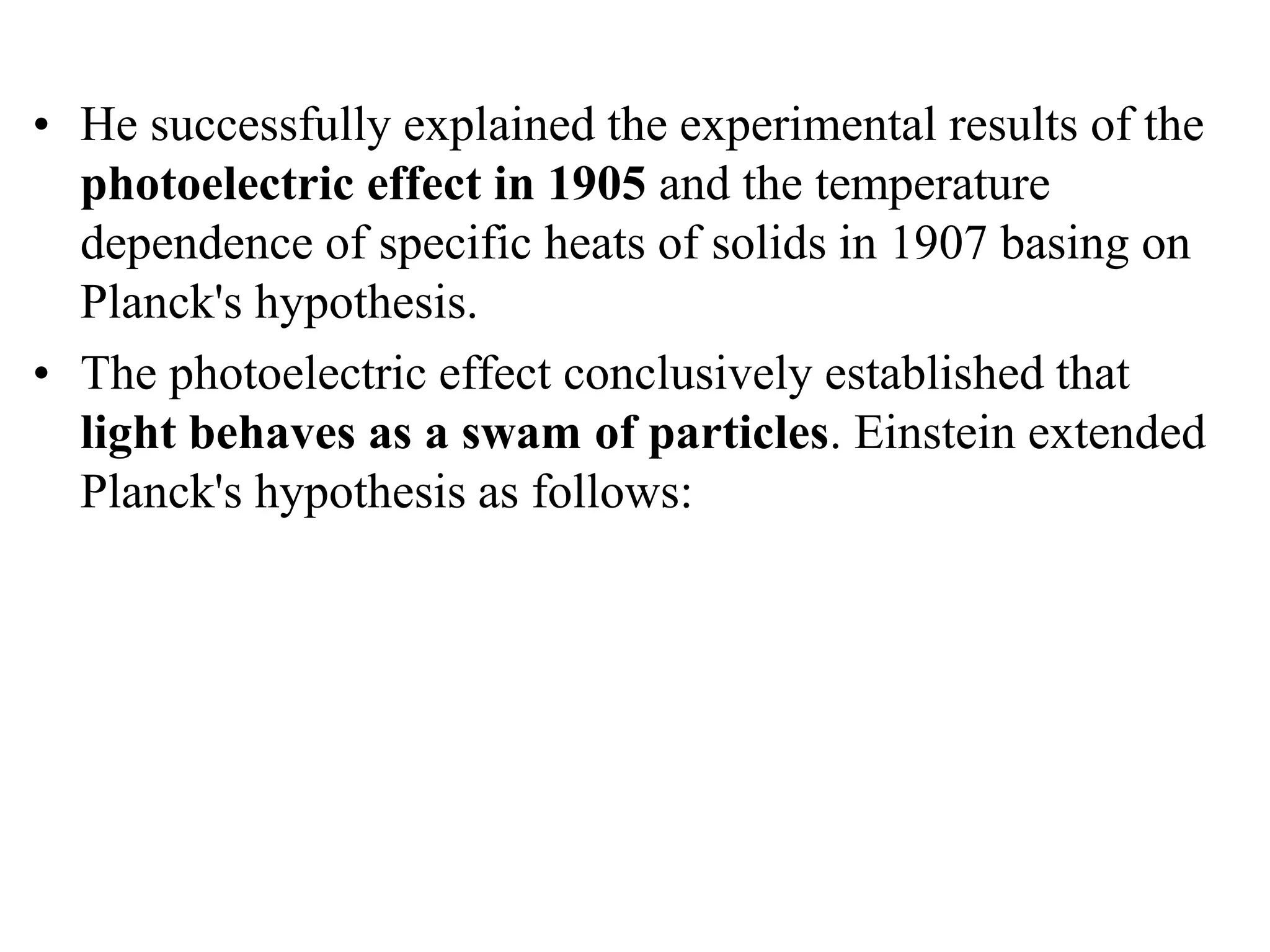
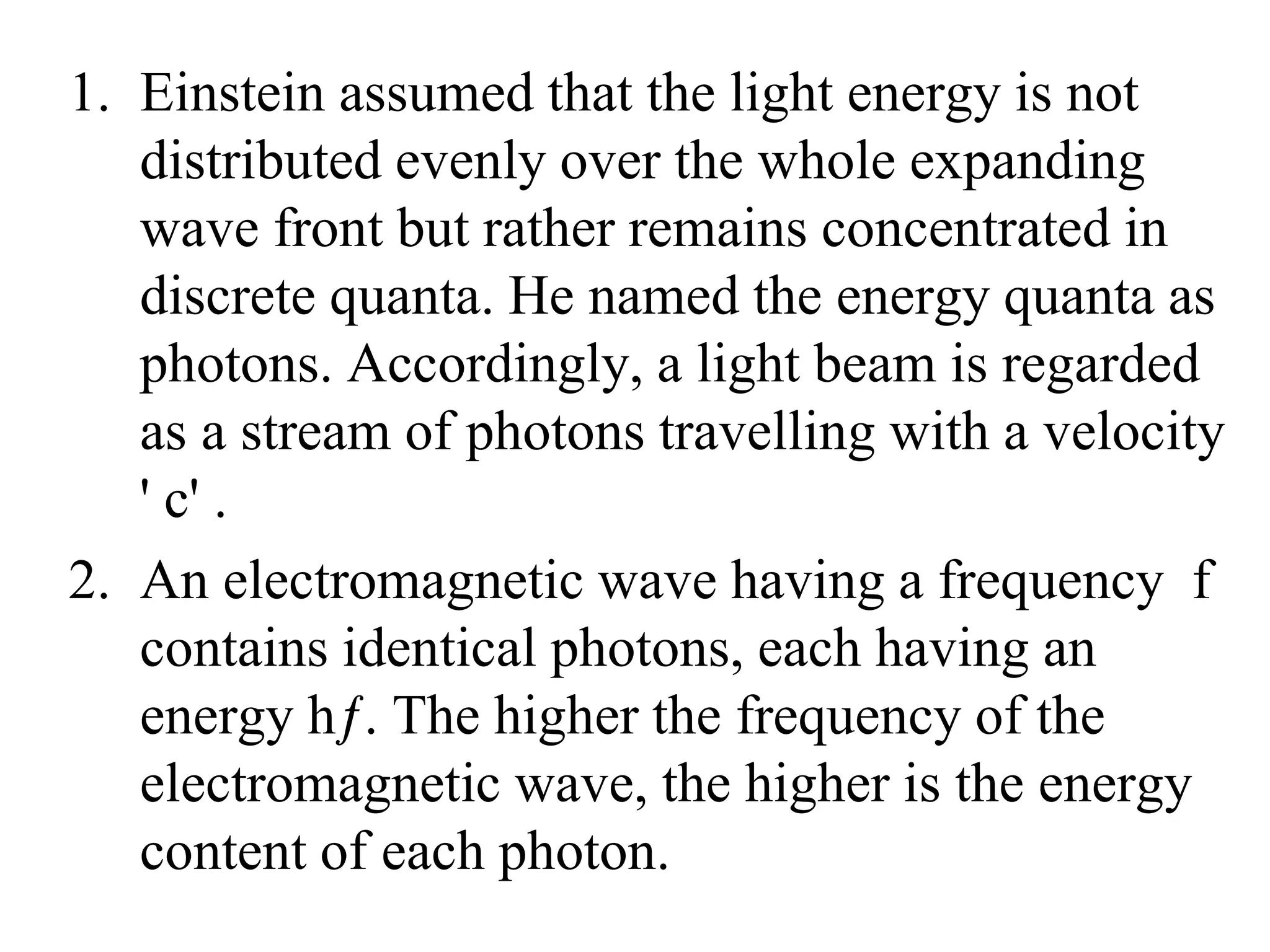
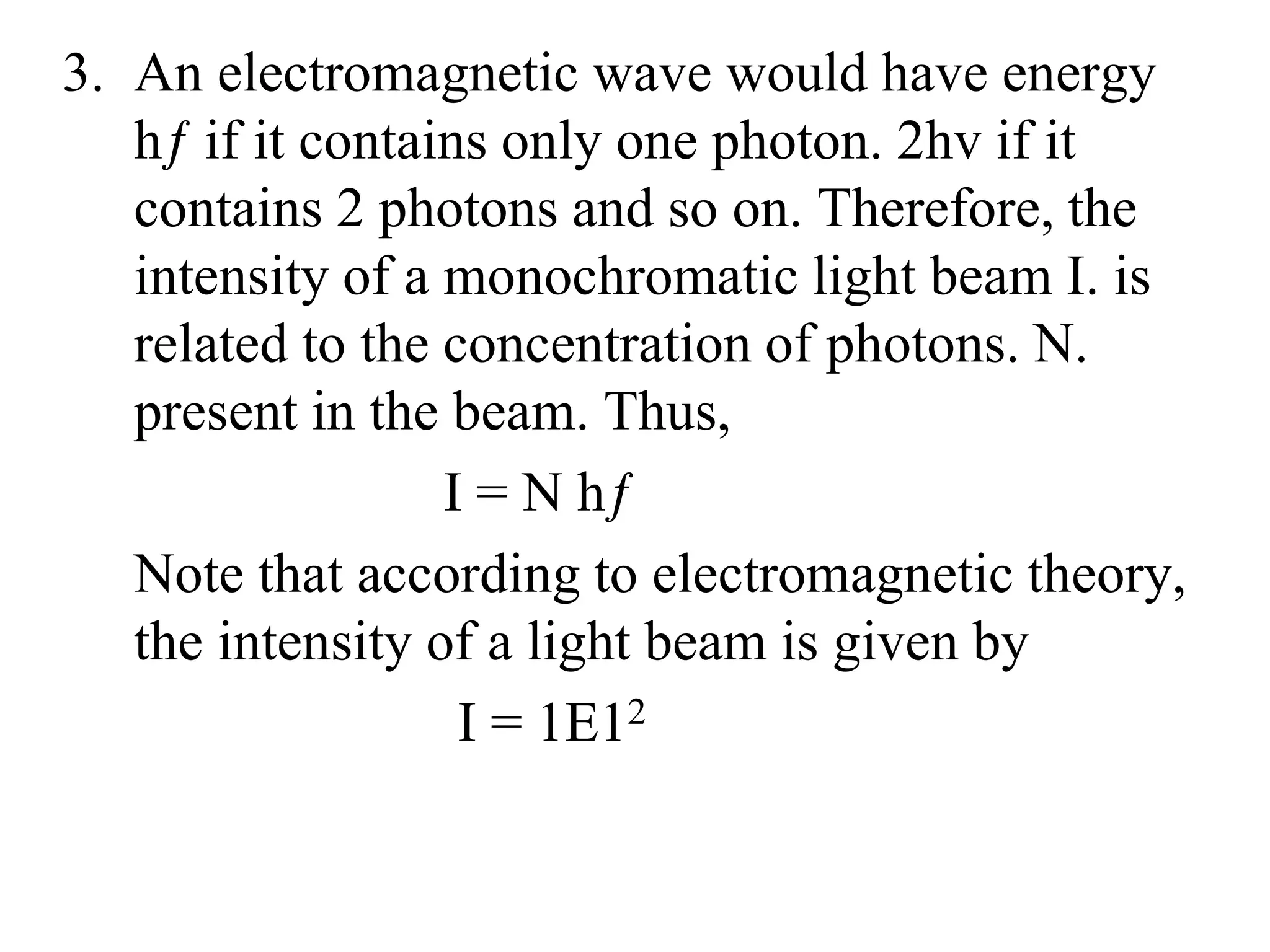
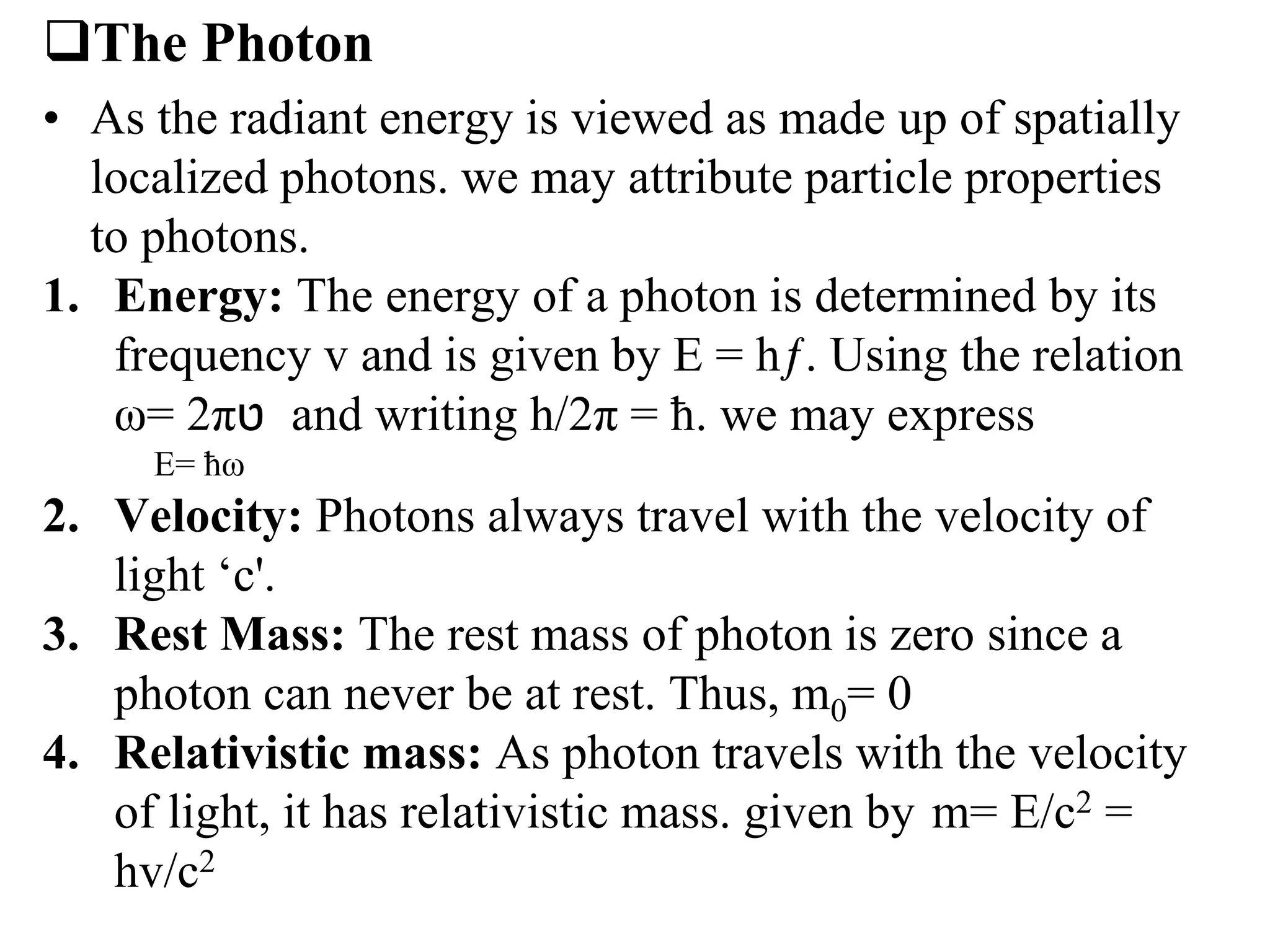
3. Quantum physics recognizes that waves and particles are less distinct than previously thought. Key insights include that light can behave as particles called photons, and particles can behave as waves.












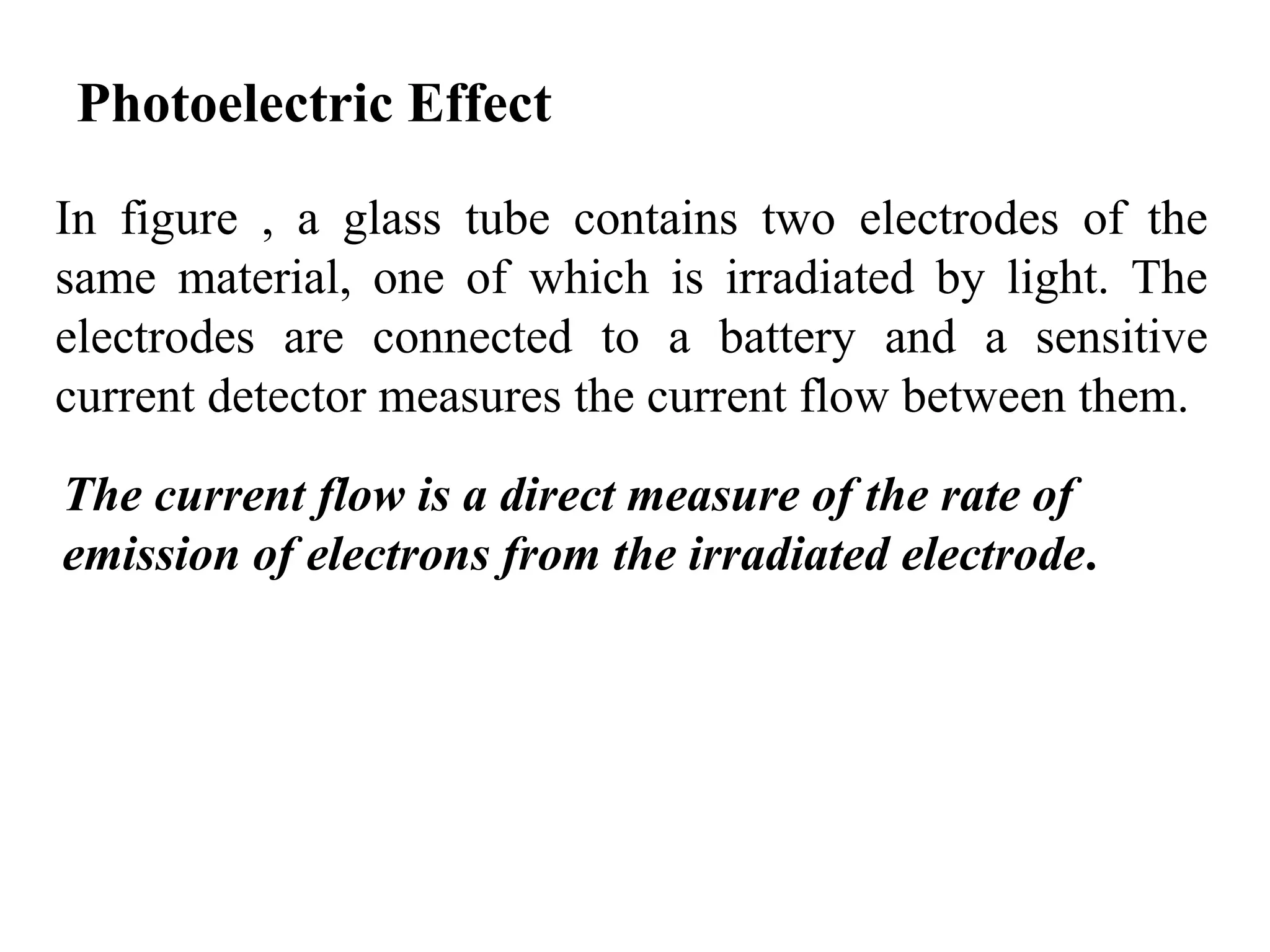


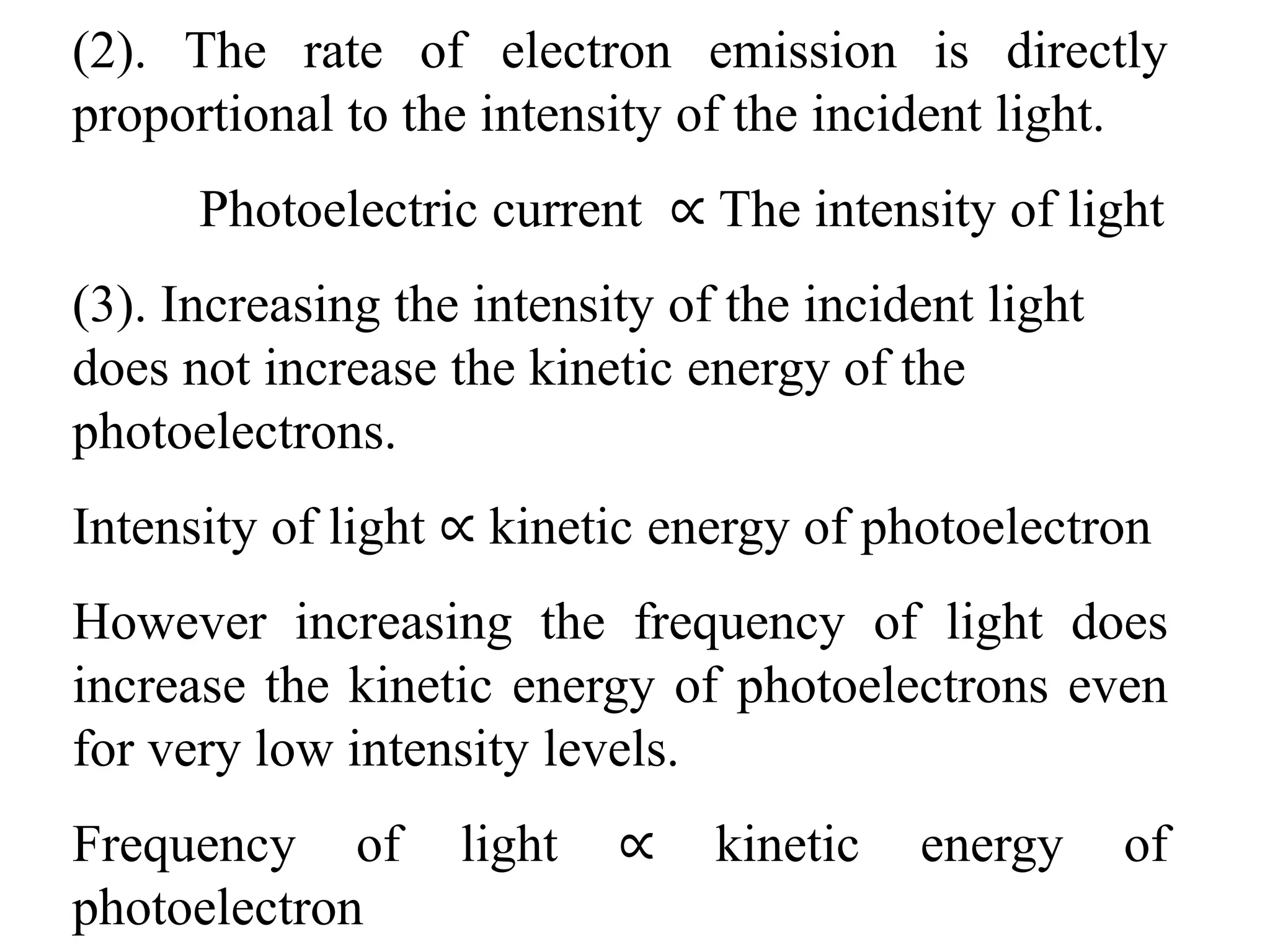
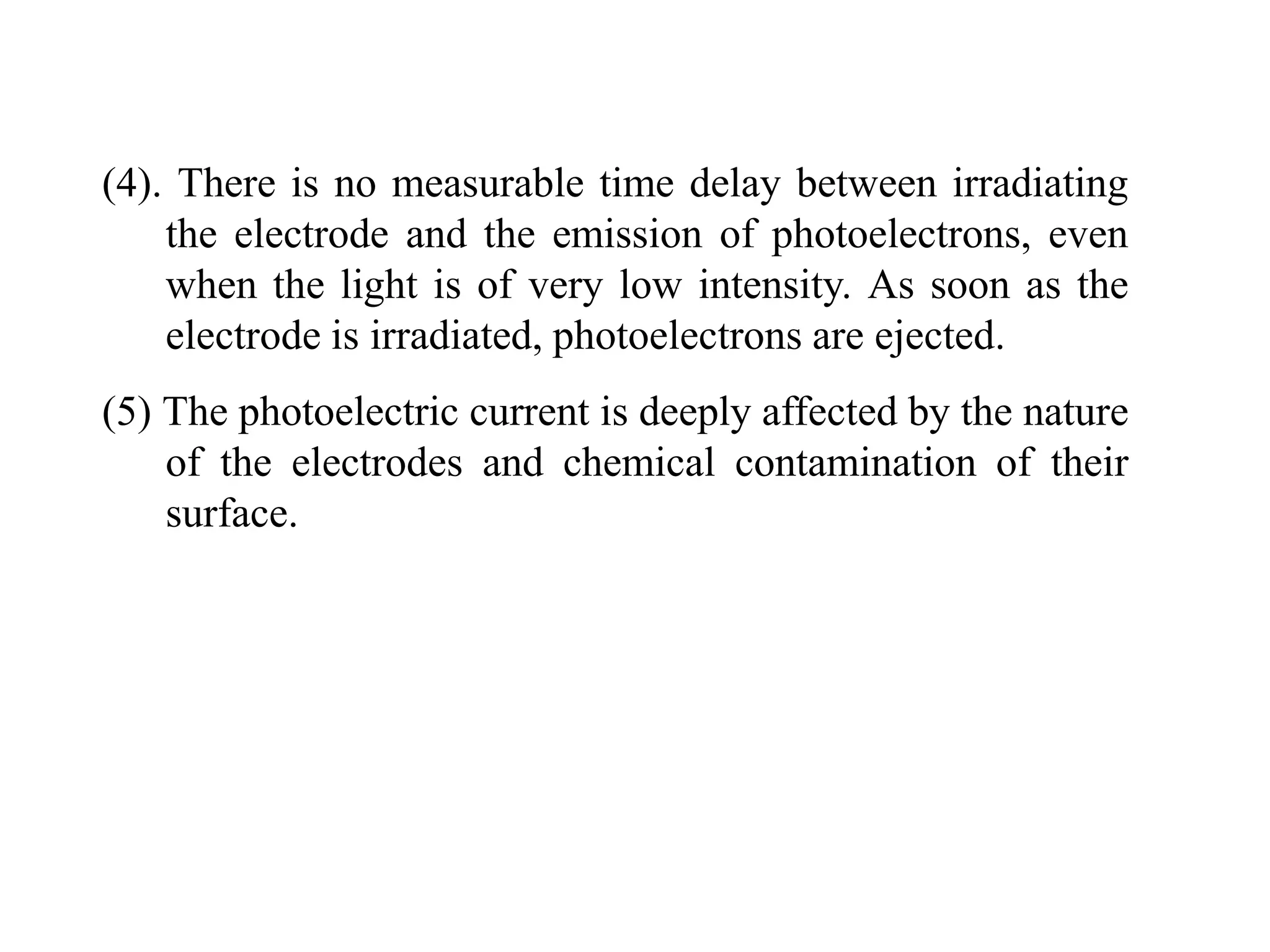
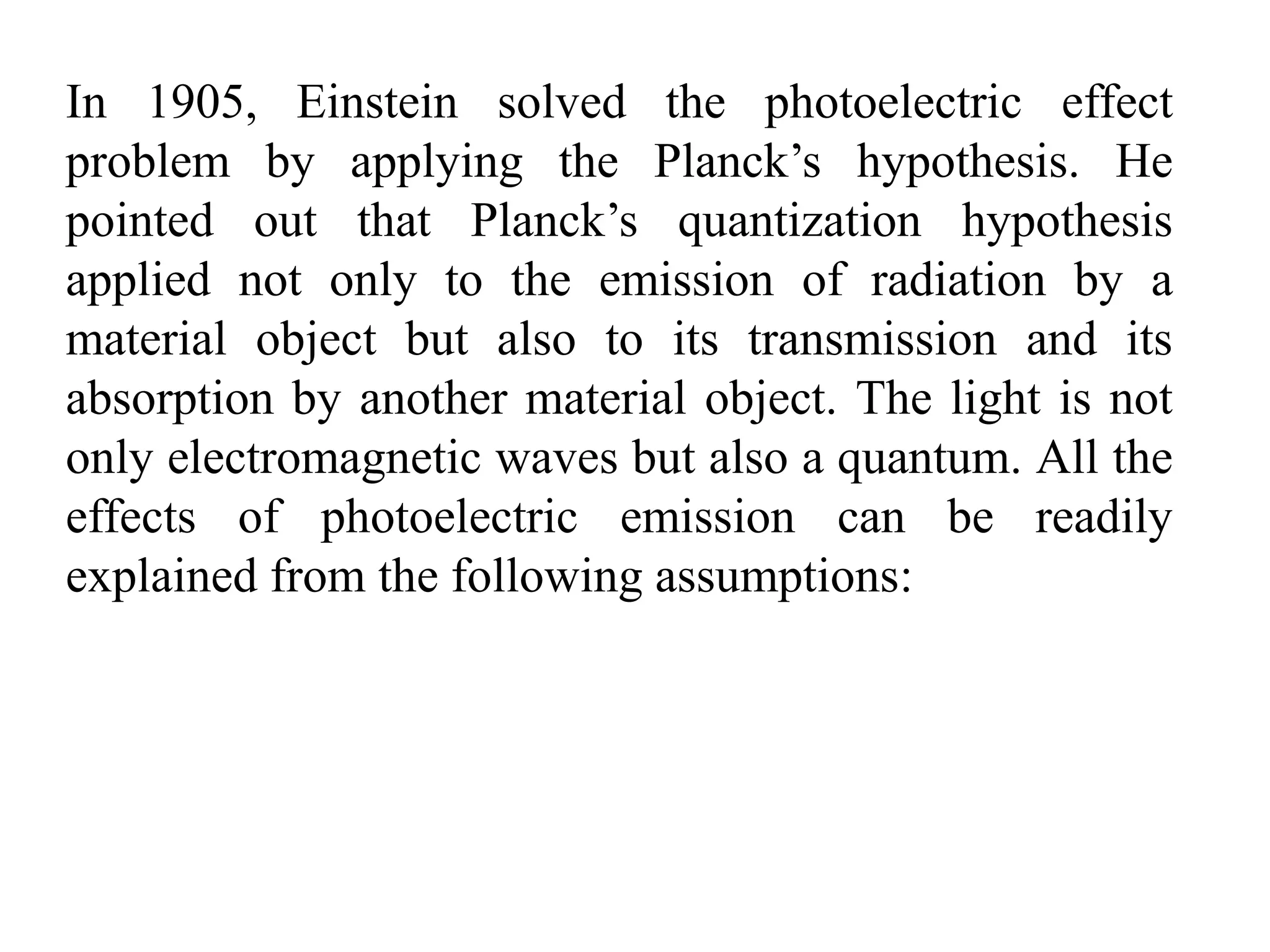
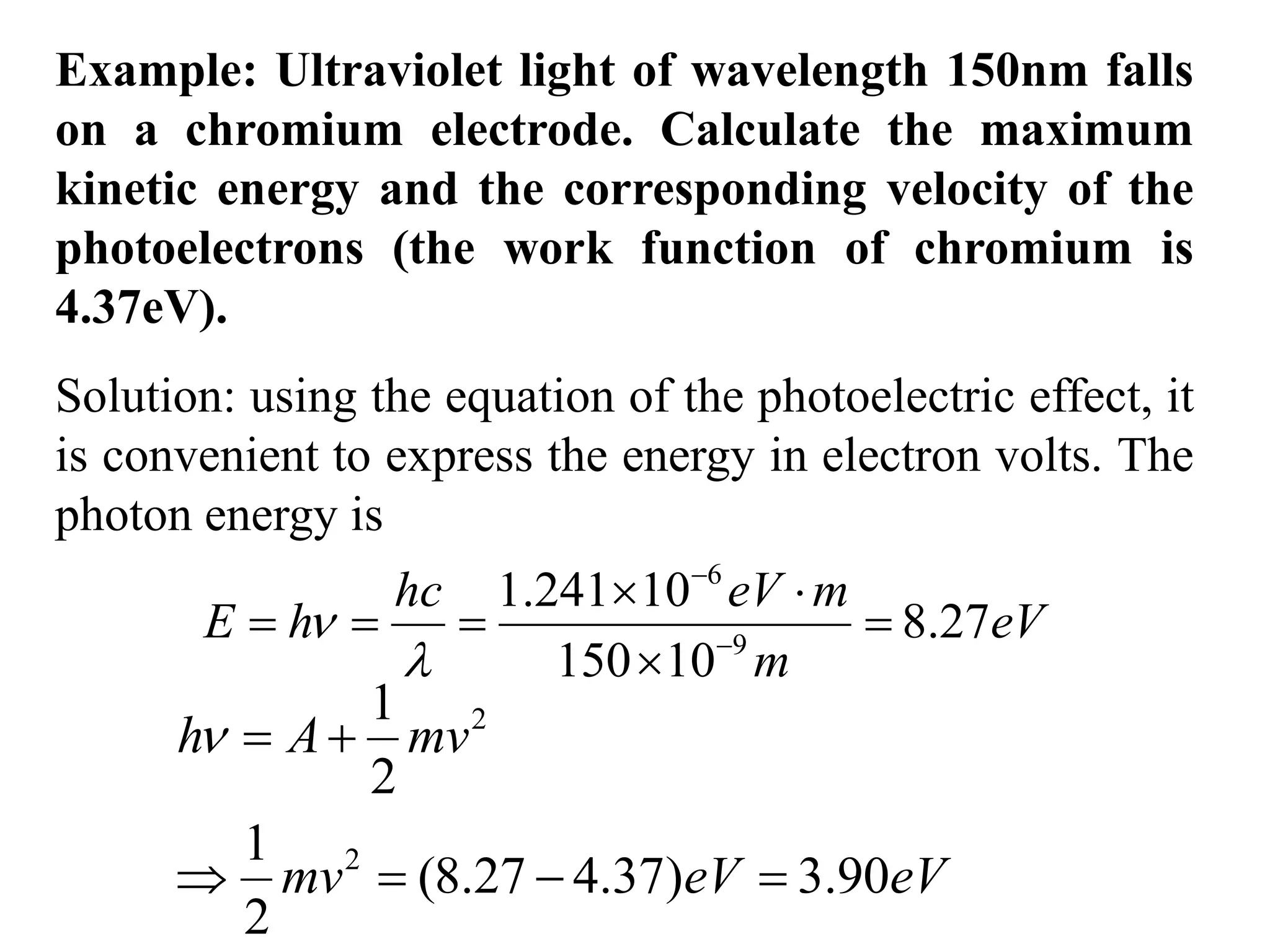
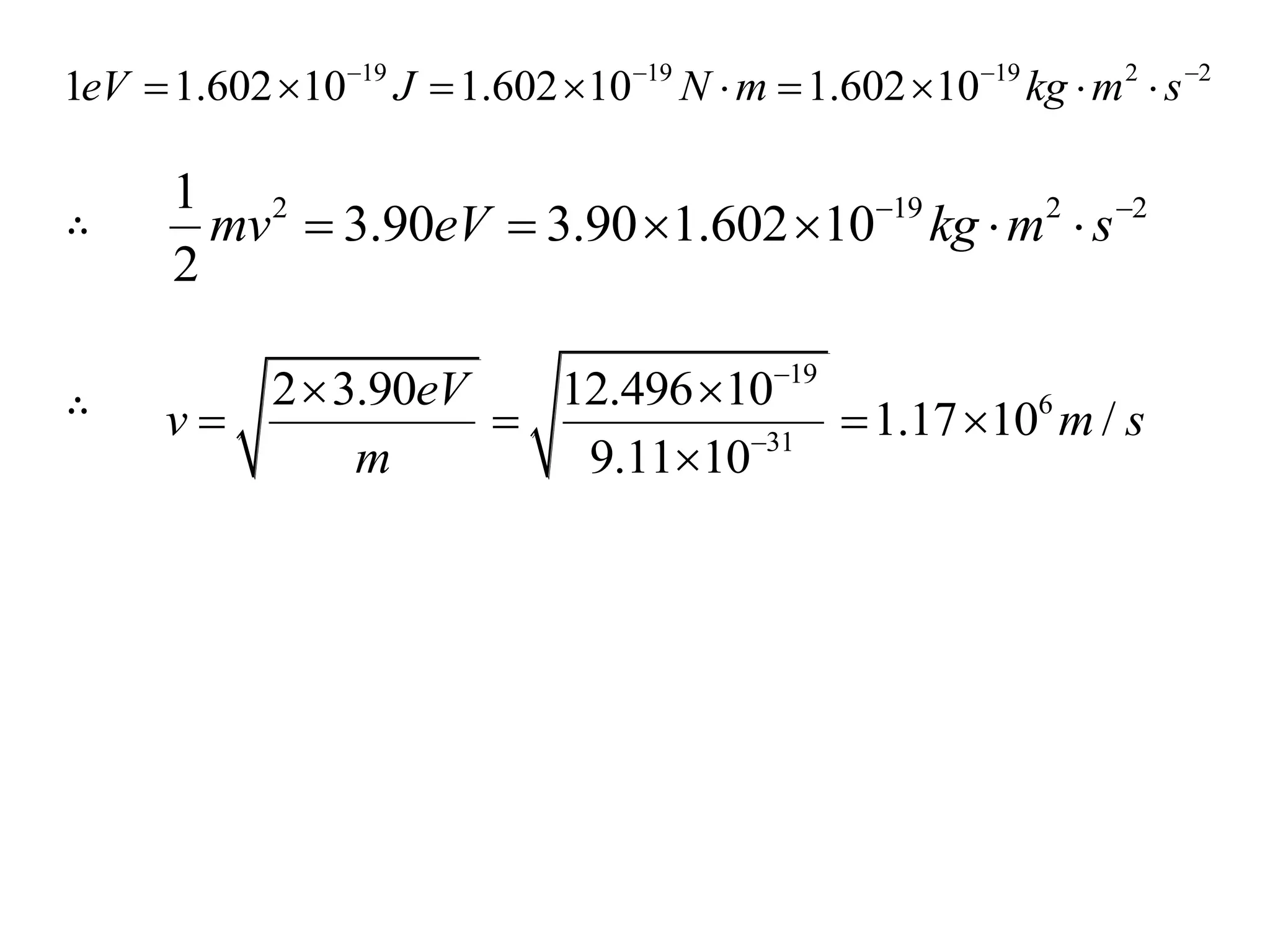

![3. Calculate the energy of a photon of blue light with a
frequency of 6.67 x 1014 Hz. (State in eV) [2.76eV]
4. Calculate the energy of a photon of red light with a
wavelength of 630 nm. [1.97eV]
5. Barium has a work function of 2.48 eV. What is the
maximum kinetic energy of the ejected electron if the
metal is illuminated by light of wavelength 450 nm?
[0.28 eV]
6. When a 350nm light ray falls on a metal, the maximum
kinetic energy of the photoelectron is 1.20eV. What is the
work function of the metal? [2.3 eV]
7. A photon has 3.3 x 10-19 J of energy. What is the
wavelength of this photon?
8. What is the energy of one quantum of 5.0 x 1014 Hz light?](https://image.slidesharecdn.com/b-150110040225-conversion-gate01/75/B-tech-sem-i-engineering-physics-u-iv-chapter-1-atomic-physics-44-2048.jpg)
