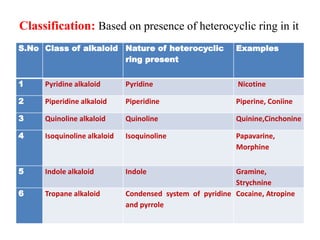
1. Alkaloids are basic nitrogenous plant compounds that contain heterocyclic rings. They are classified based on the heterocyclic ring present and have pharmacological activity.
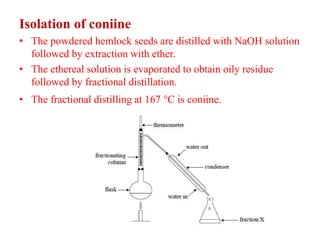
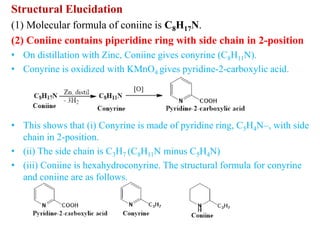
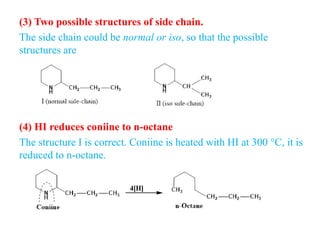
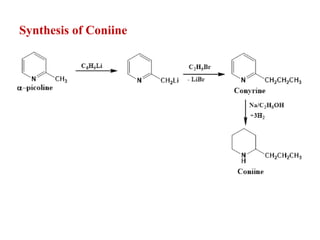
2. Coniine is a colorless, toxic alkaloid found in hemlock seeds. It contains a piperidine ring and was used to kill Socrates. Its structure was determined to be hexahydroconyrine.
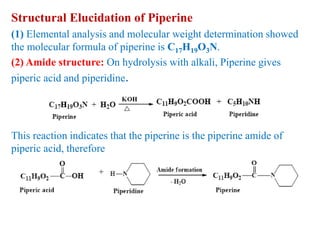
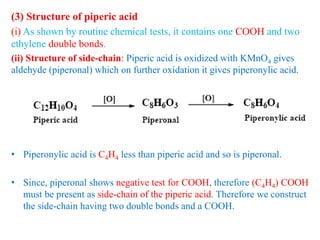
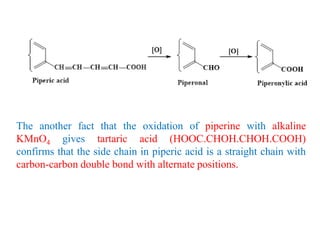
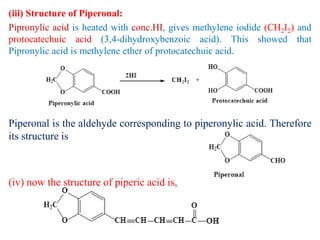
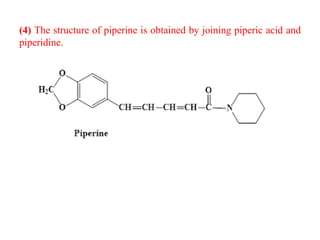
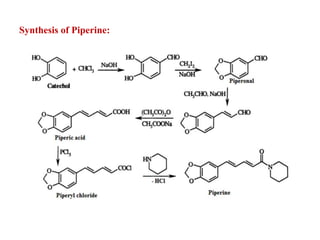
3. Piperine is a weak base found in black pepper. It contains a piperidine group joined to piperic acid, which has a side chain containing two carbon-carbon double bonds and a carboxyl group. Its structure was elucidated through chemical reactions.