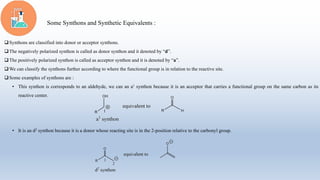
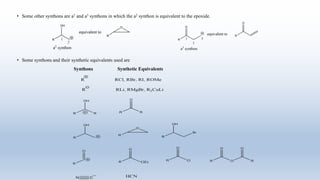
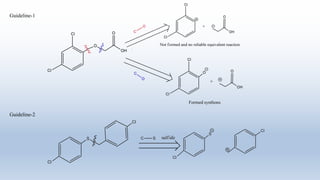
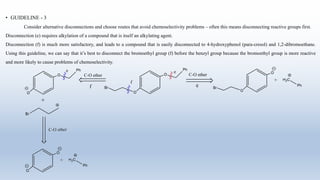
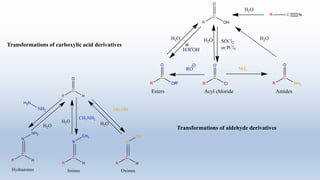
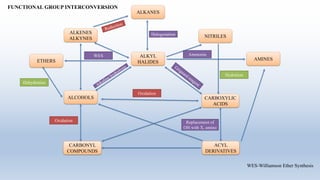
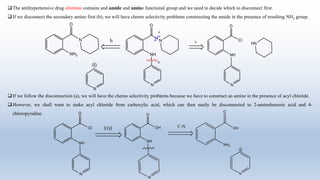
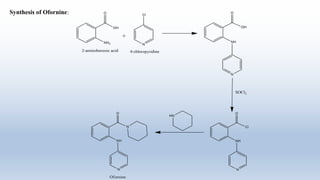
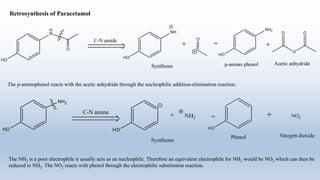
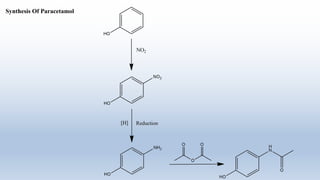
This document provides an introduction to retrosynthesis, which involves working backwards from a target molecule to devise a synthetic route. It discusses key terminology like disconnections, synthons, and functional group interconversions. The principles of retrosynthesis are disconnection, where an imaginary bond cleavage corresponds to a synthetic reaction, and functional group interconversion, which involves changing one functional group to another. Examples of retrosynthesis are provided for drugs like ofornine and paracetamol to illustrate these concepts.