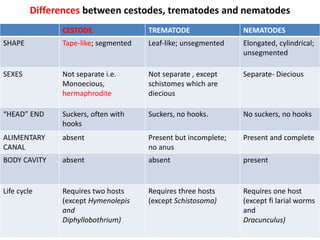
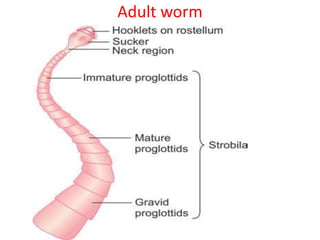
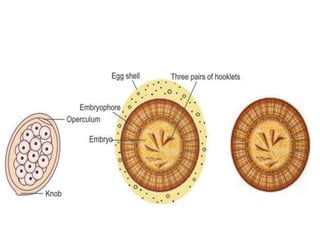
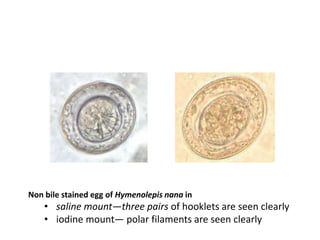
- Cestodes are tapeworm parasites that infect the intestines or tissues of hosts. They are classified as either pseudophyllidean or cyclophyllidean based on morphology and life cycle.
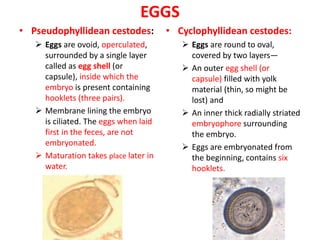
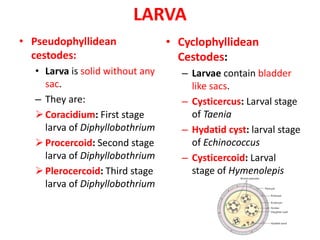
- Pseudophyllidean cestodes have unbranched uteri and operculated eggs, while cyclophyllidean cestodes have branched uteri, non-operculated eggs, and larvae that form cysts or bladders.
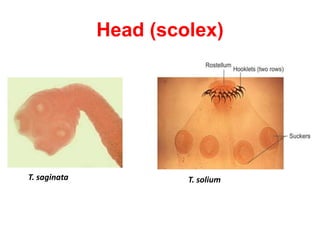
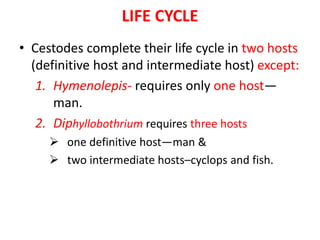
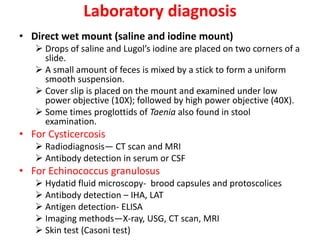
- Common cyclophyllidean cestodes include Taenia saginata, T. solium, and Echinococcus granulosus, which infect humans and require one or two intermediate hosts to complete their life cycles.