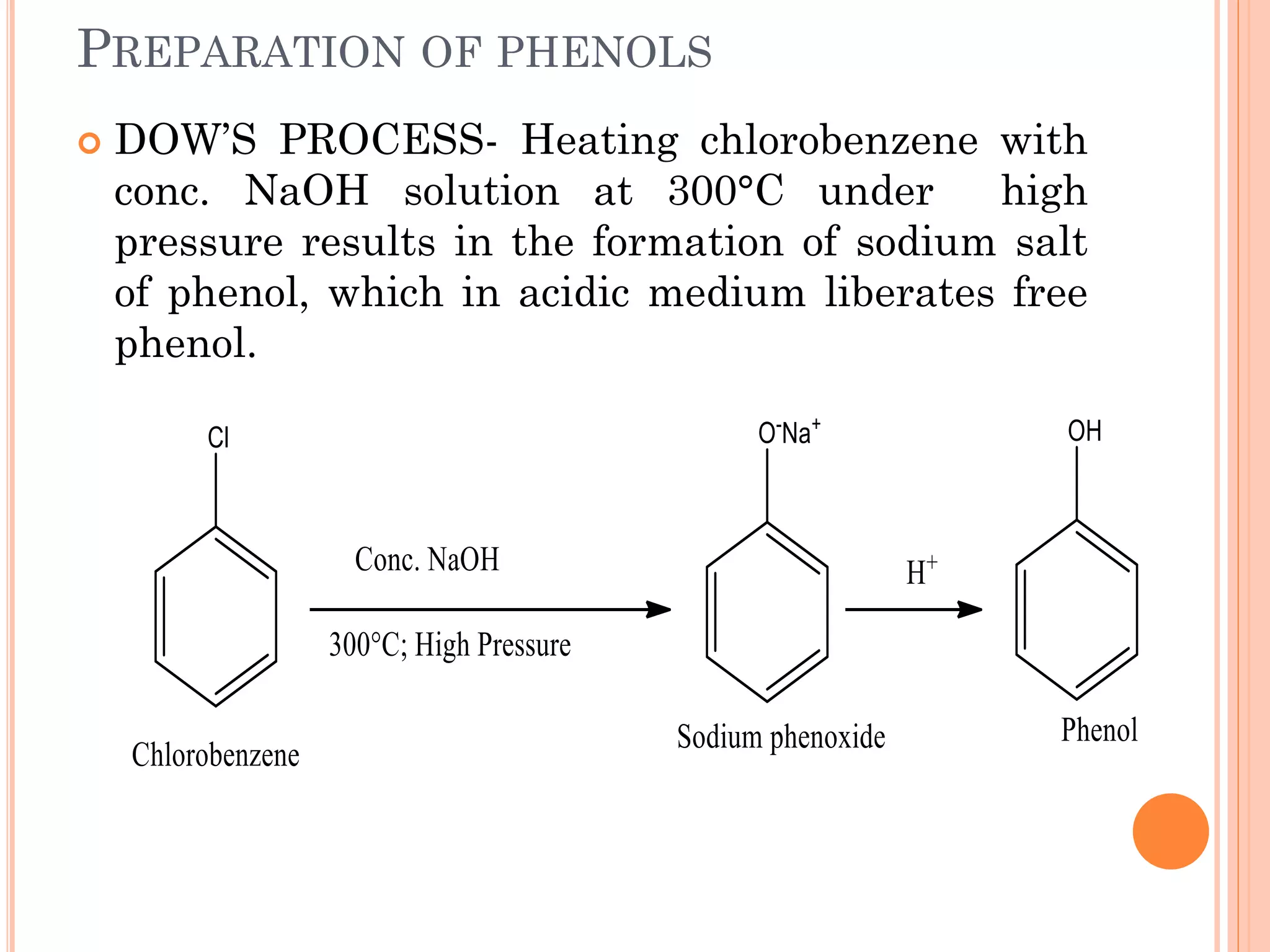
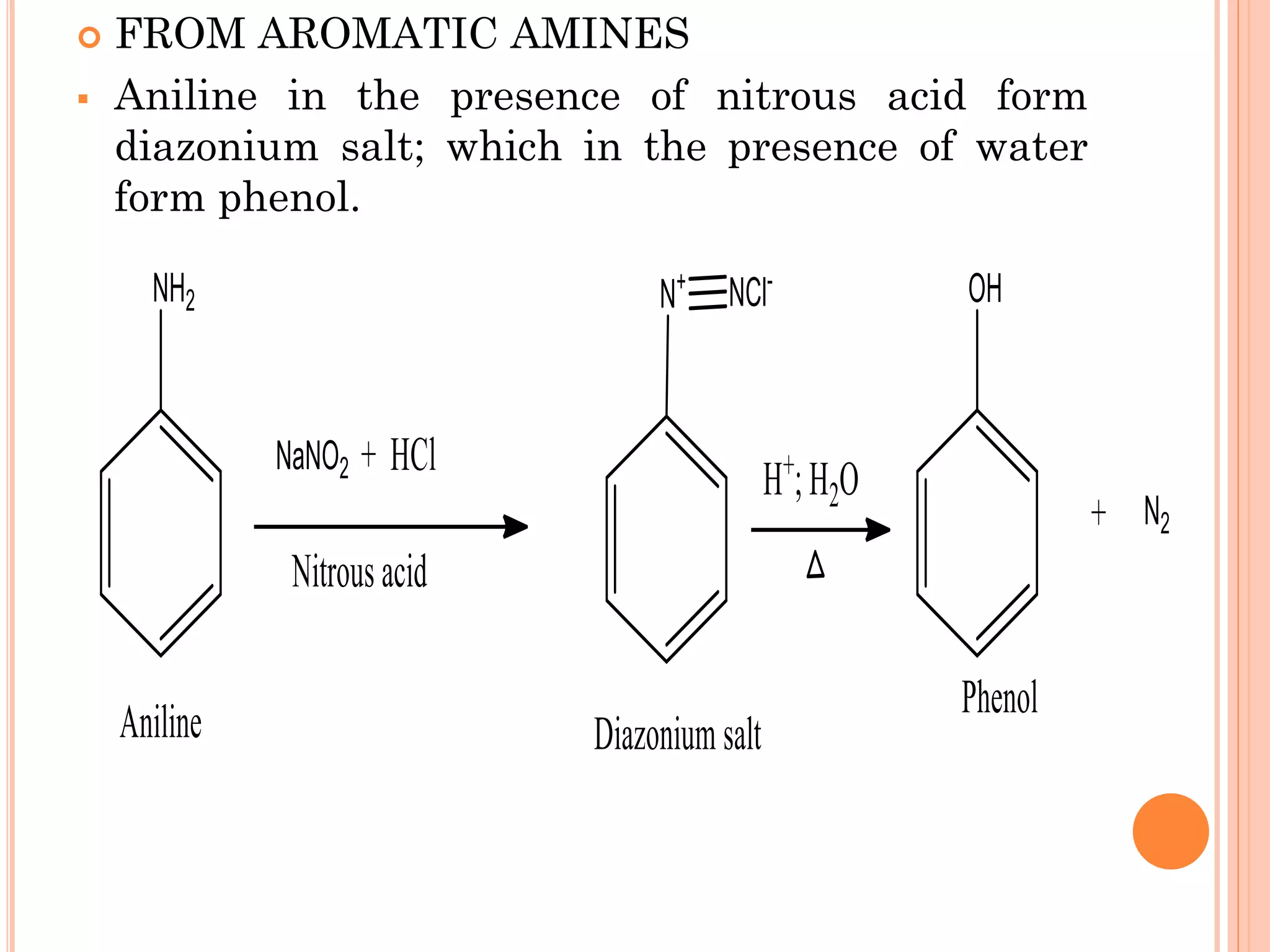
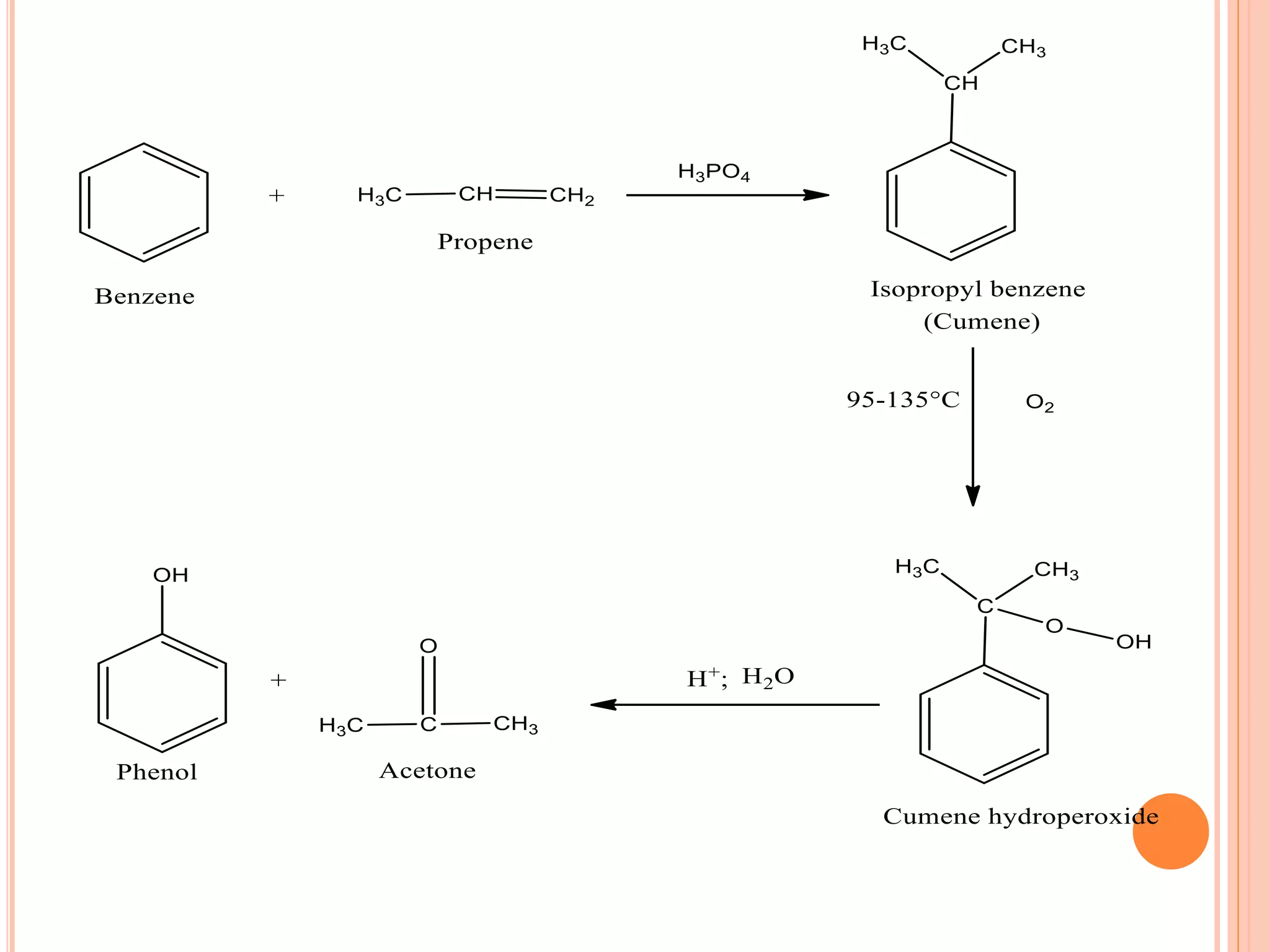
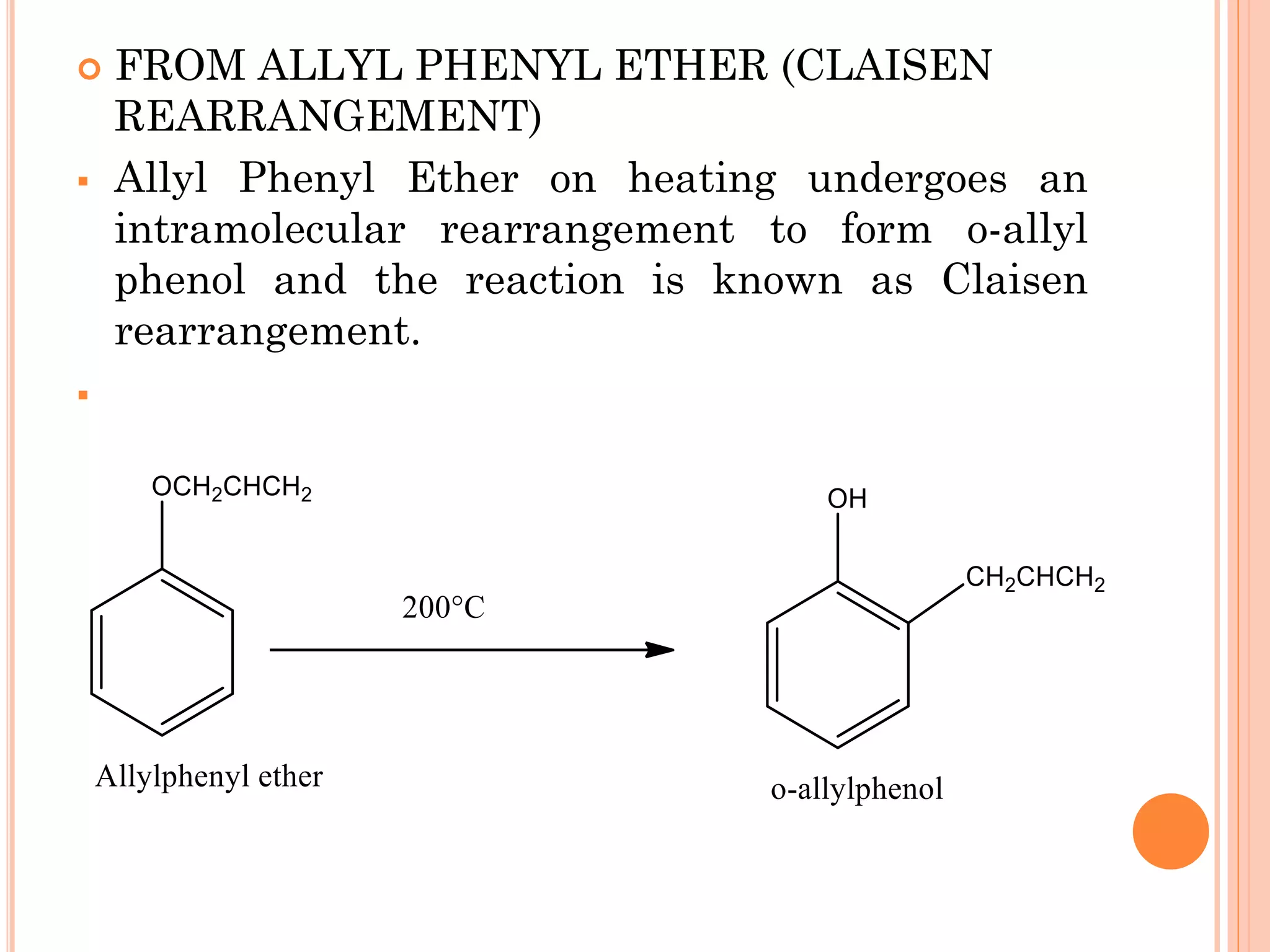
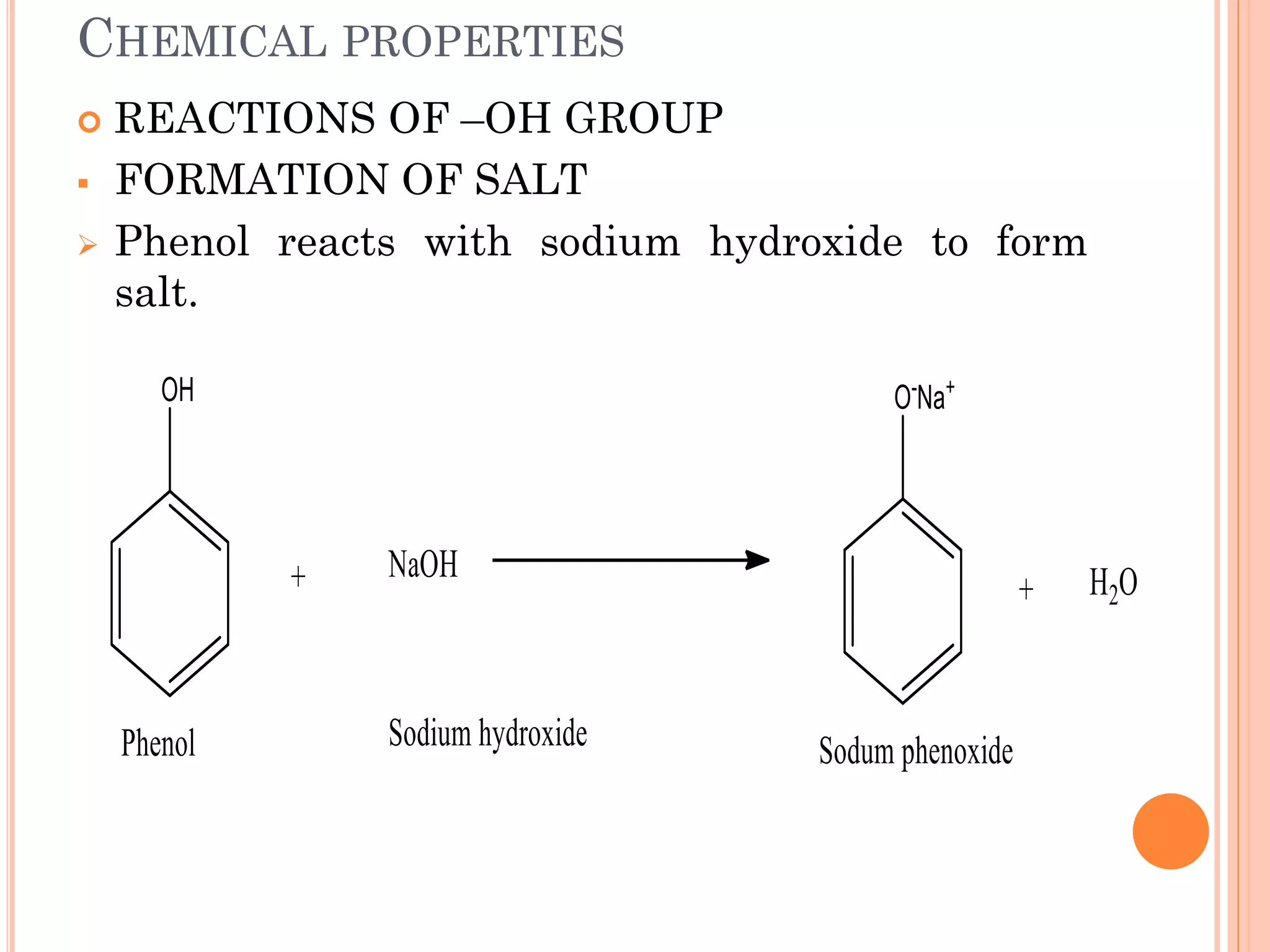
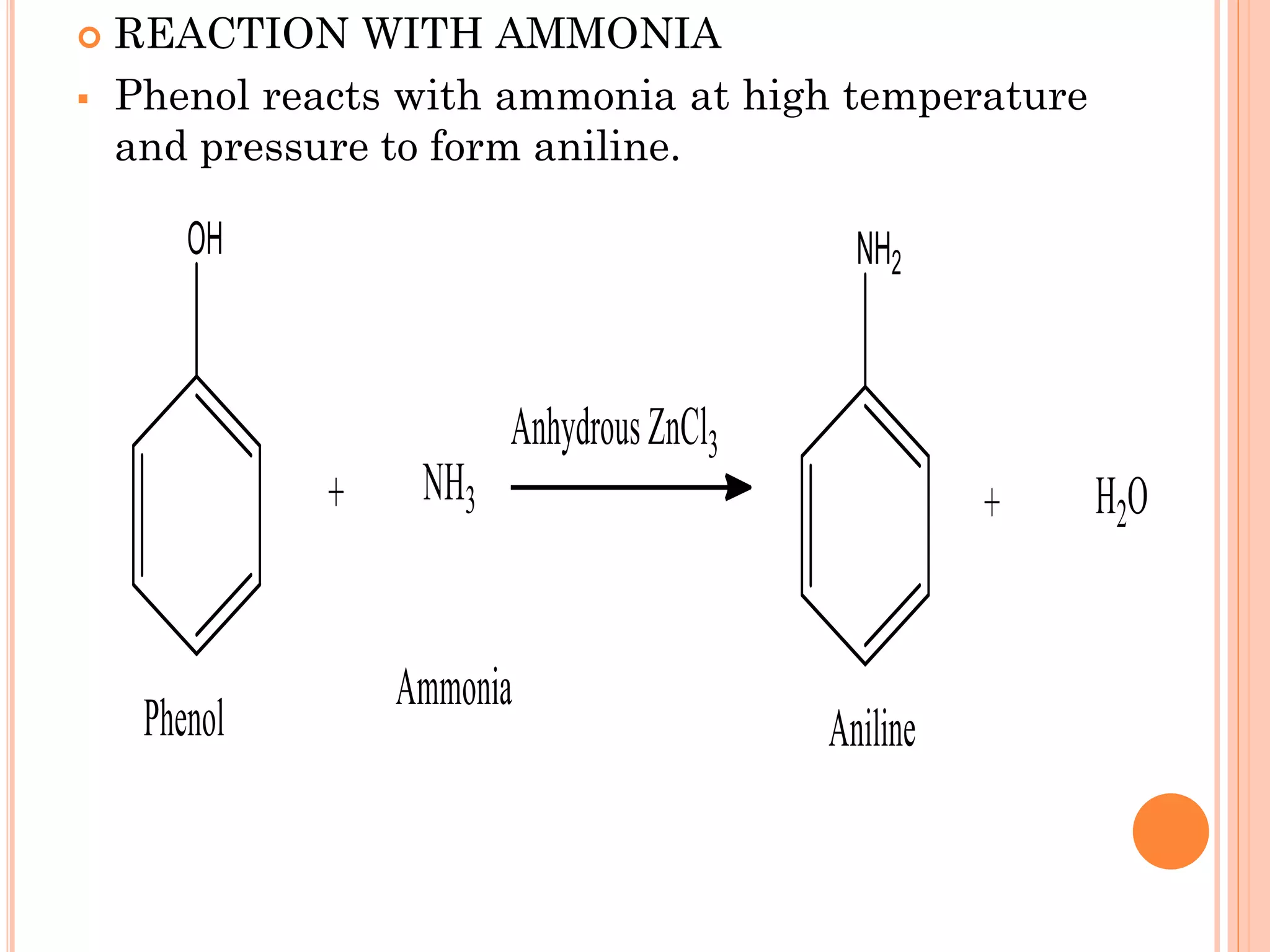
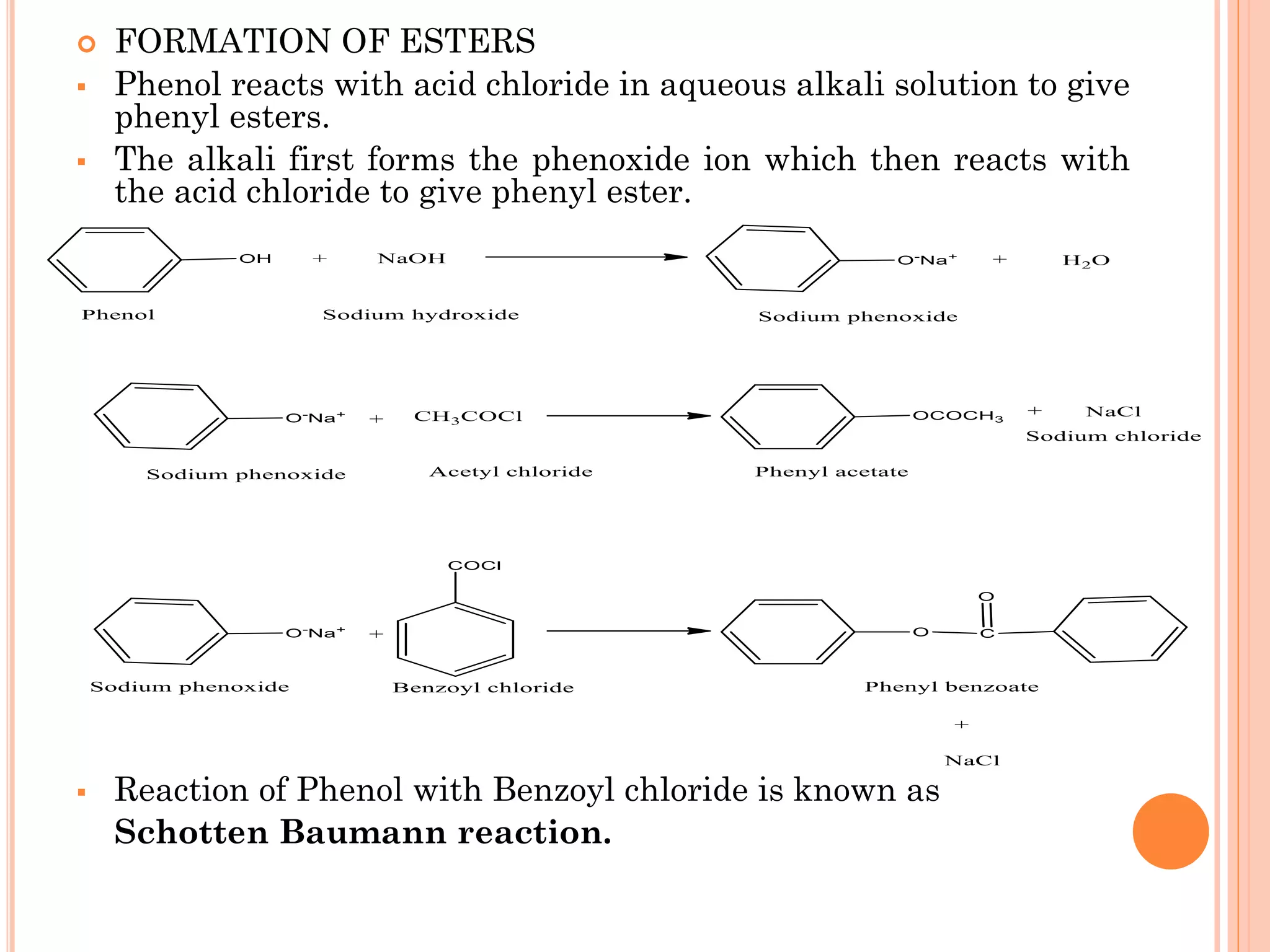
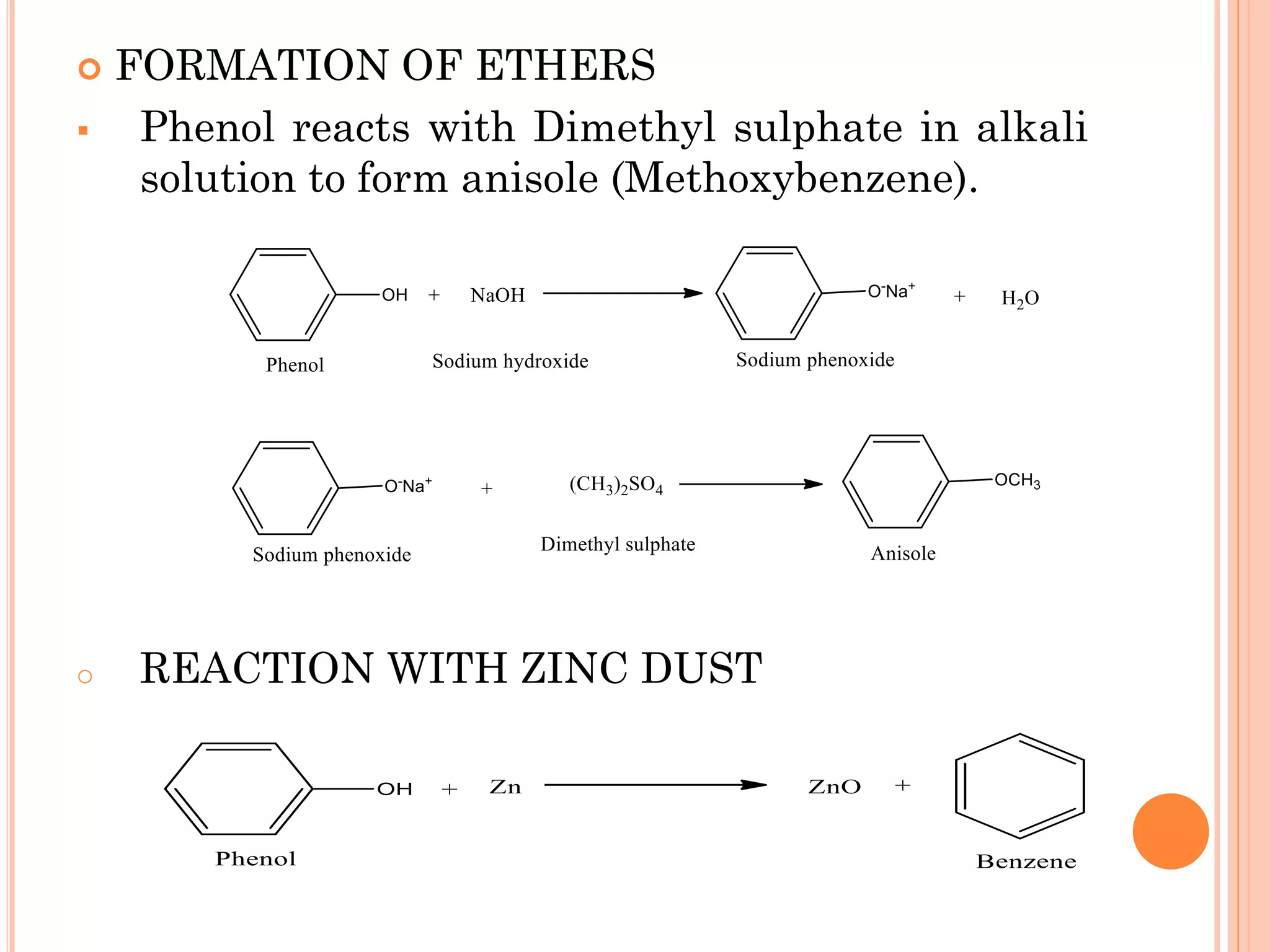
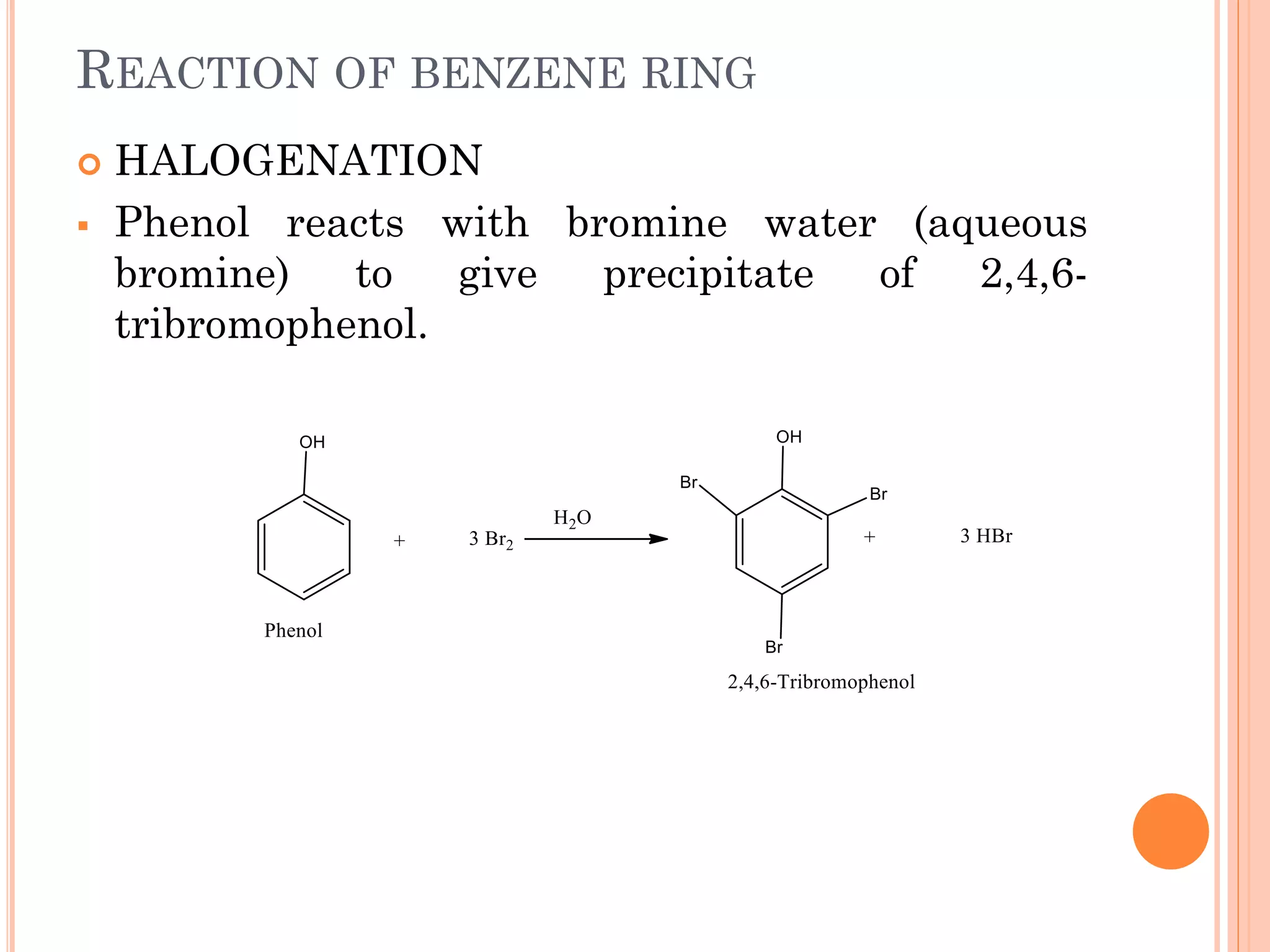
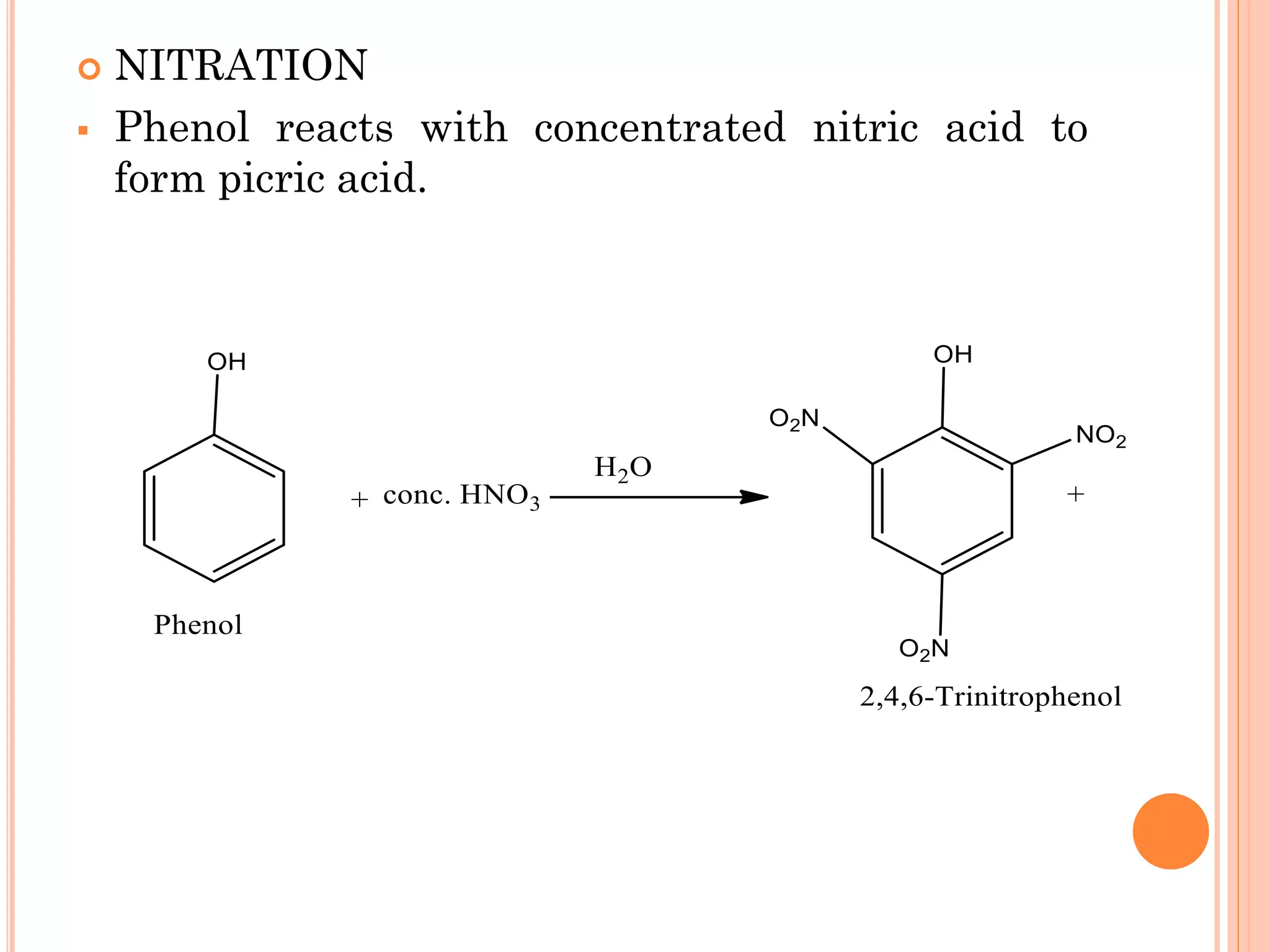
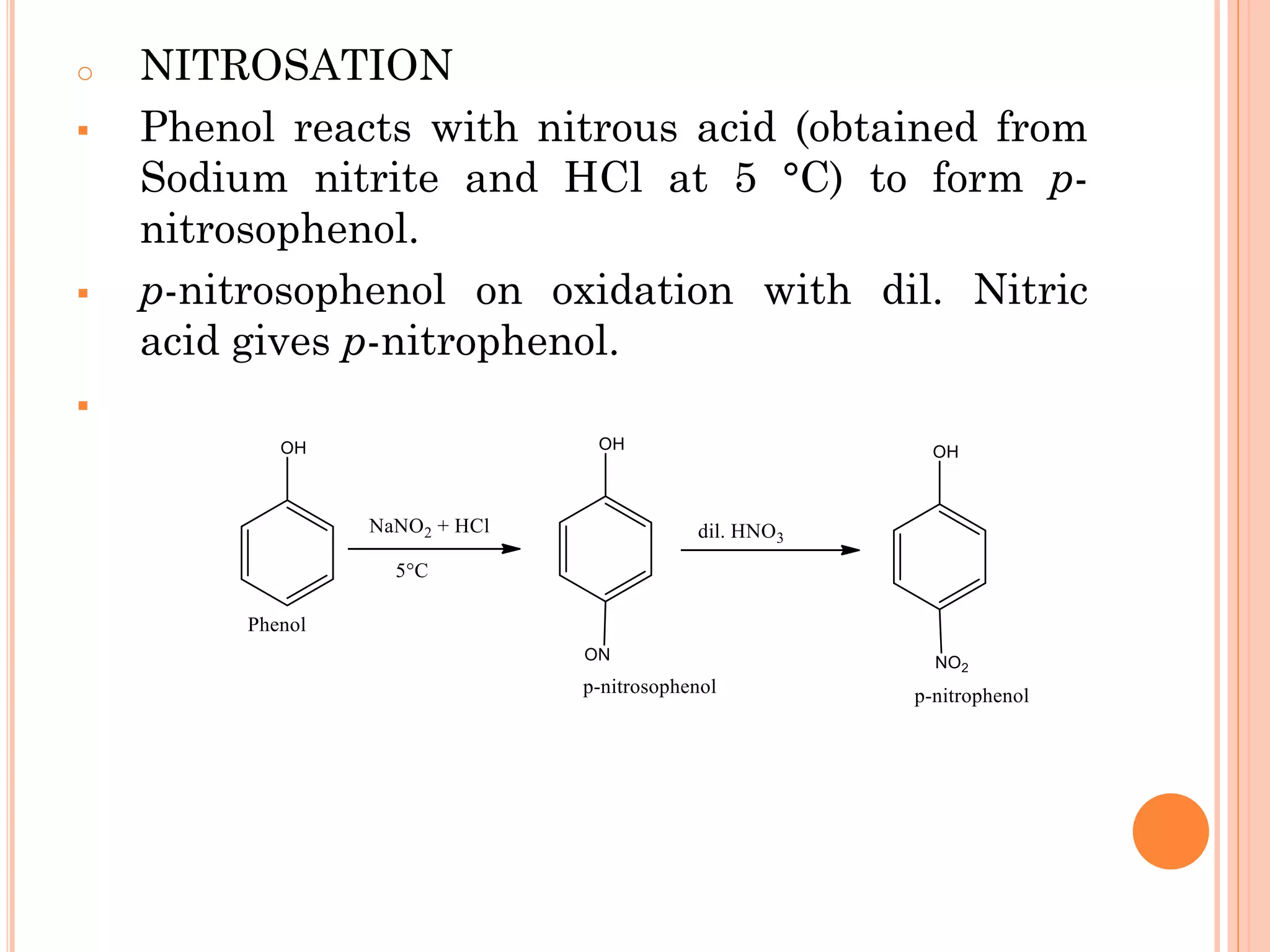
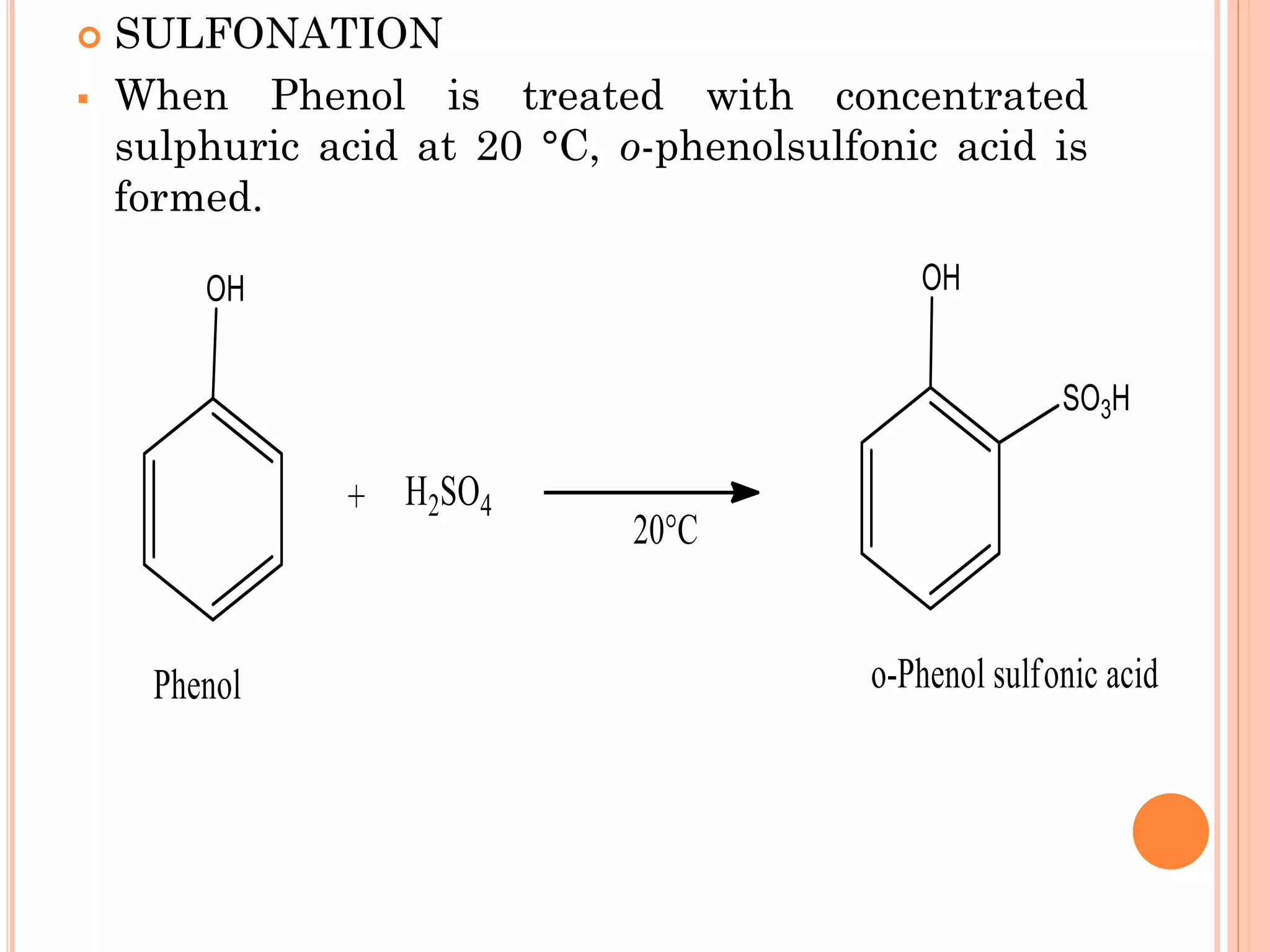
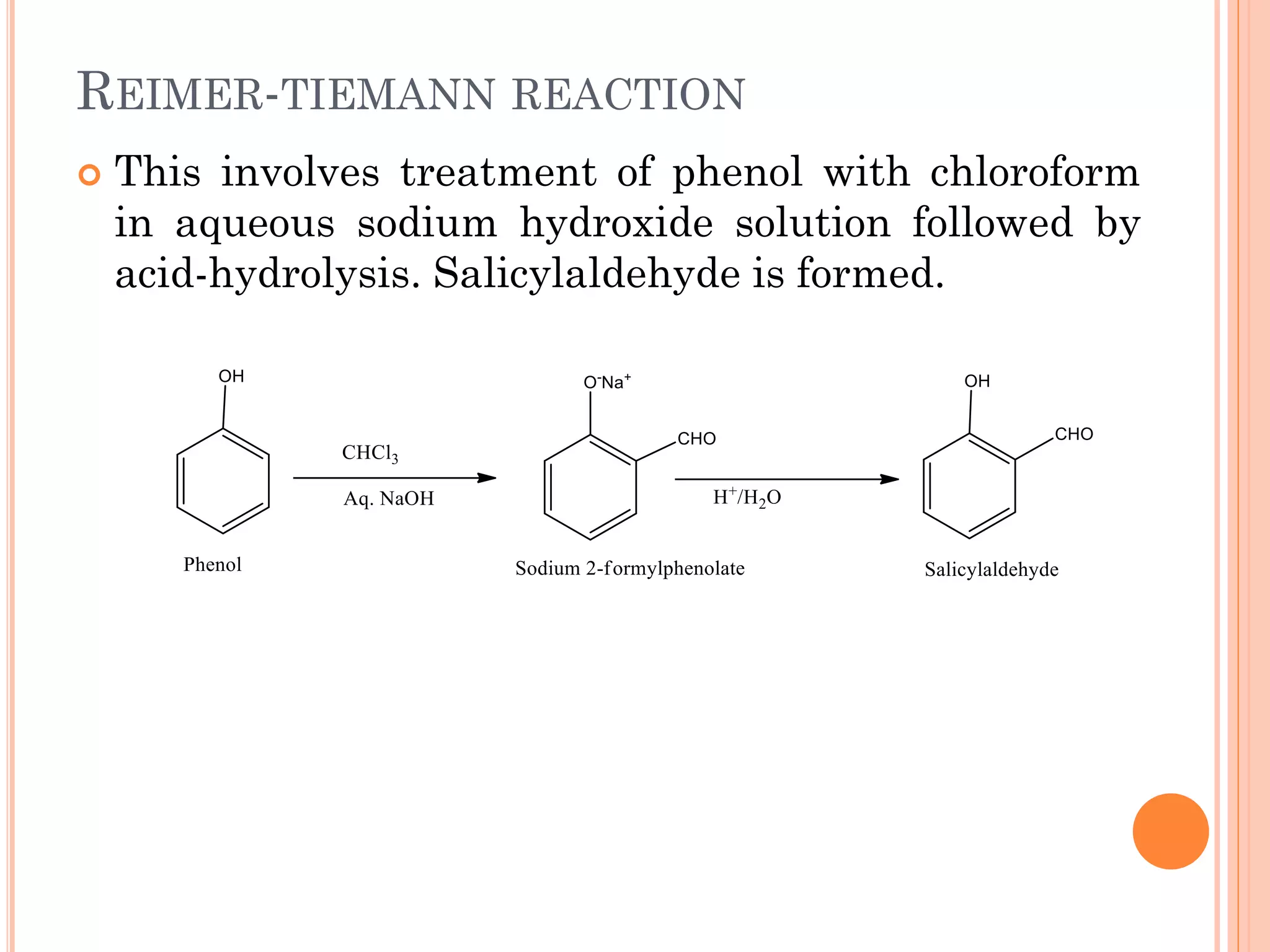
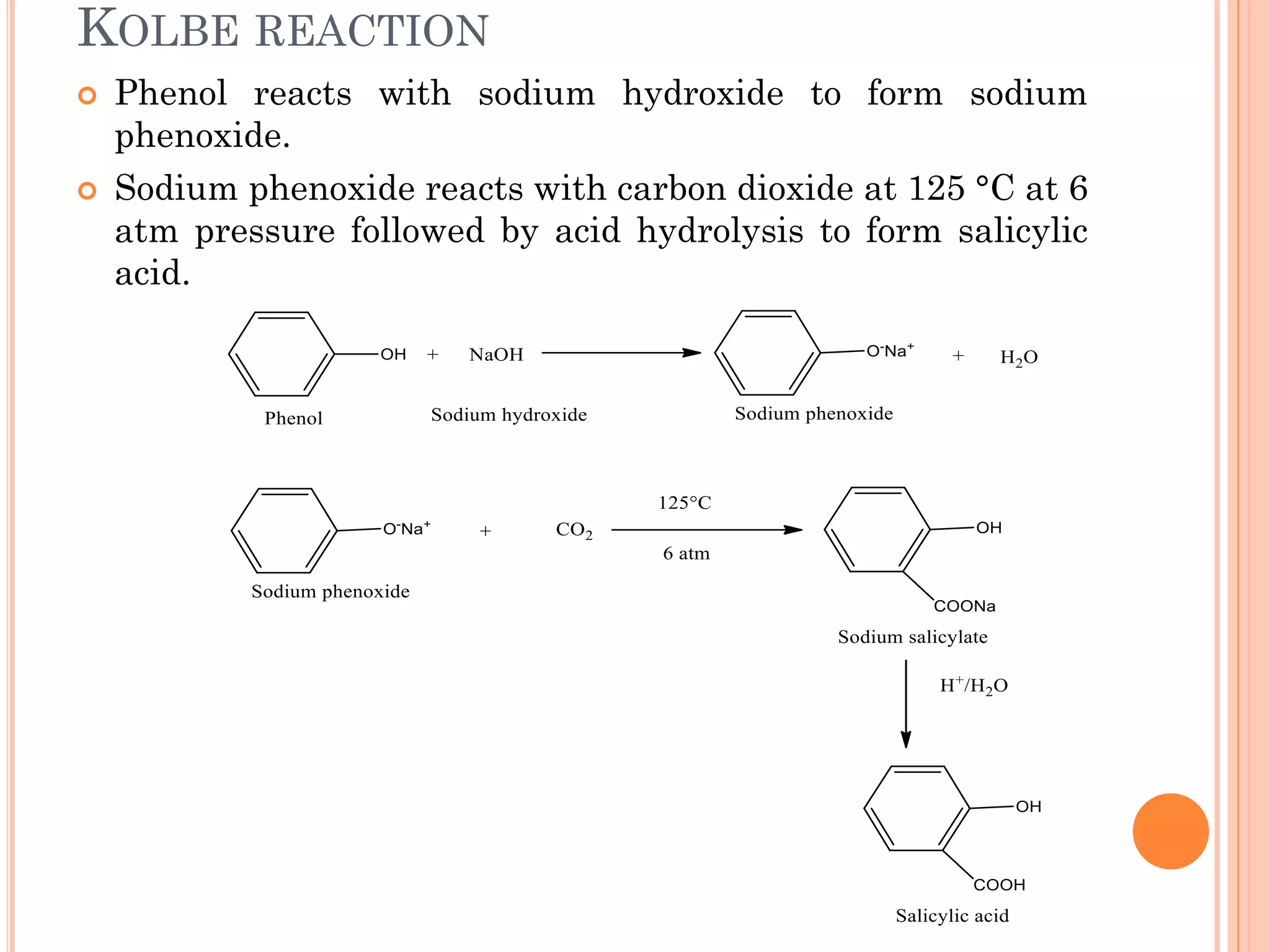
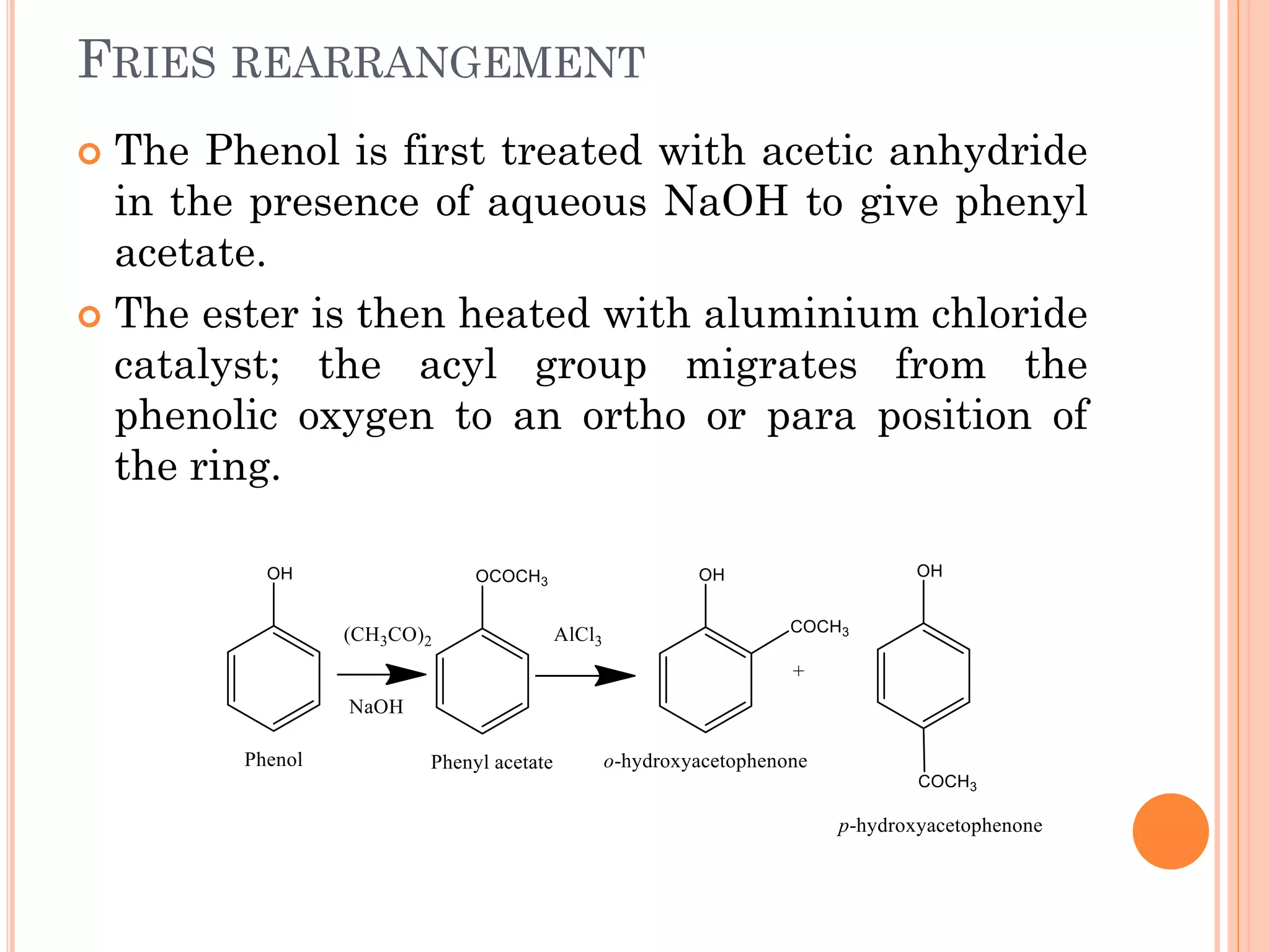
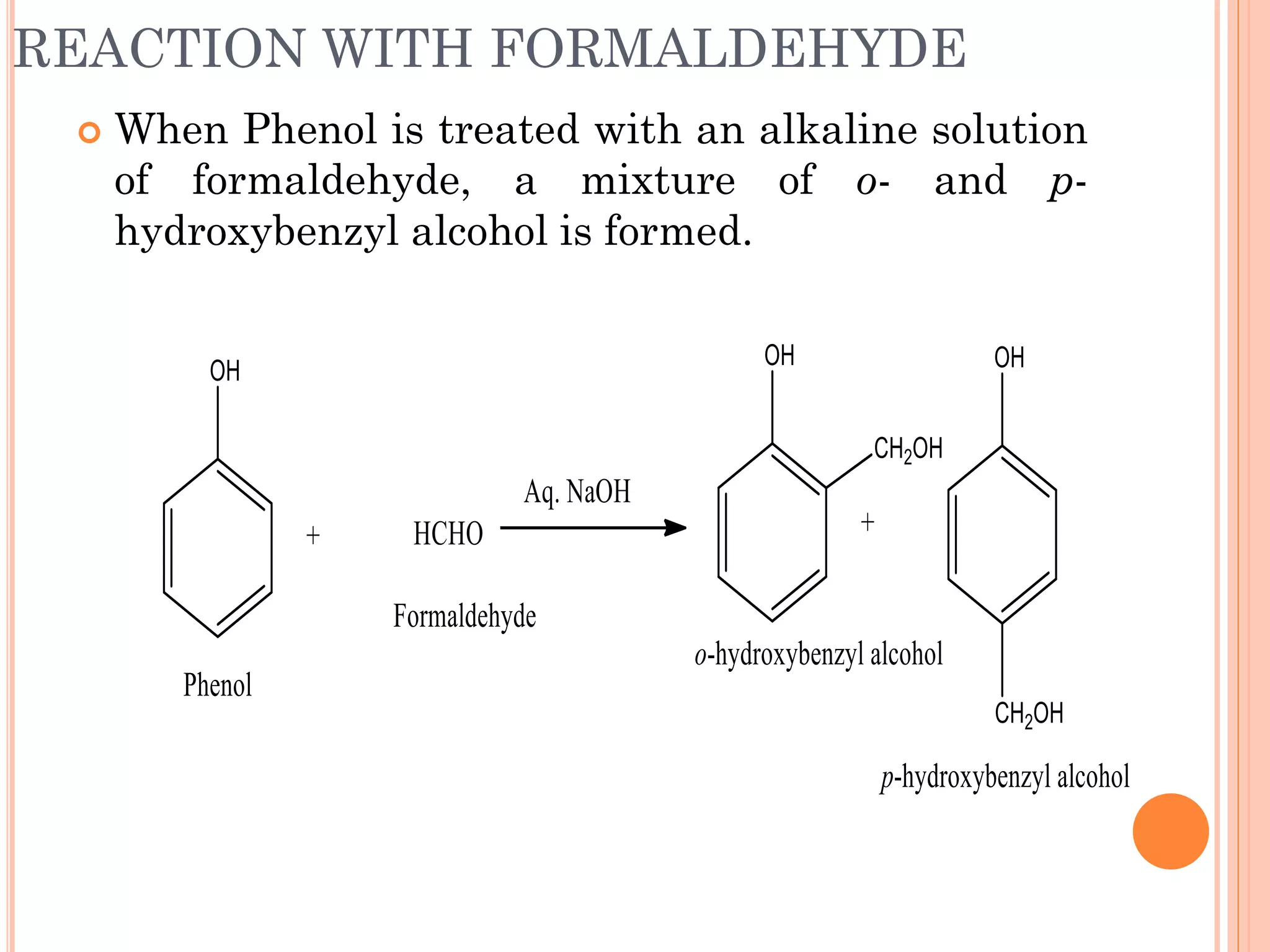
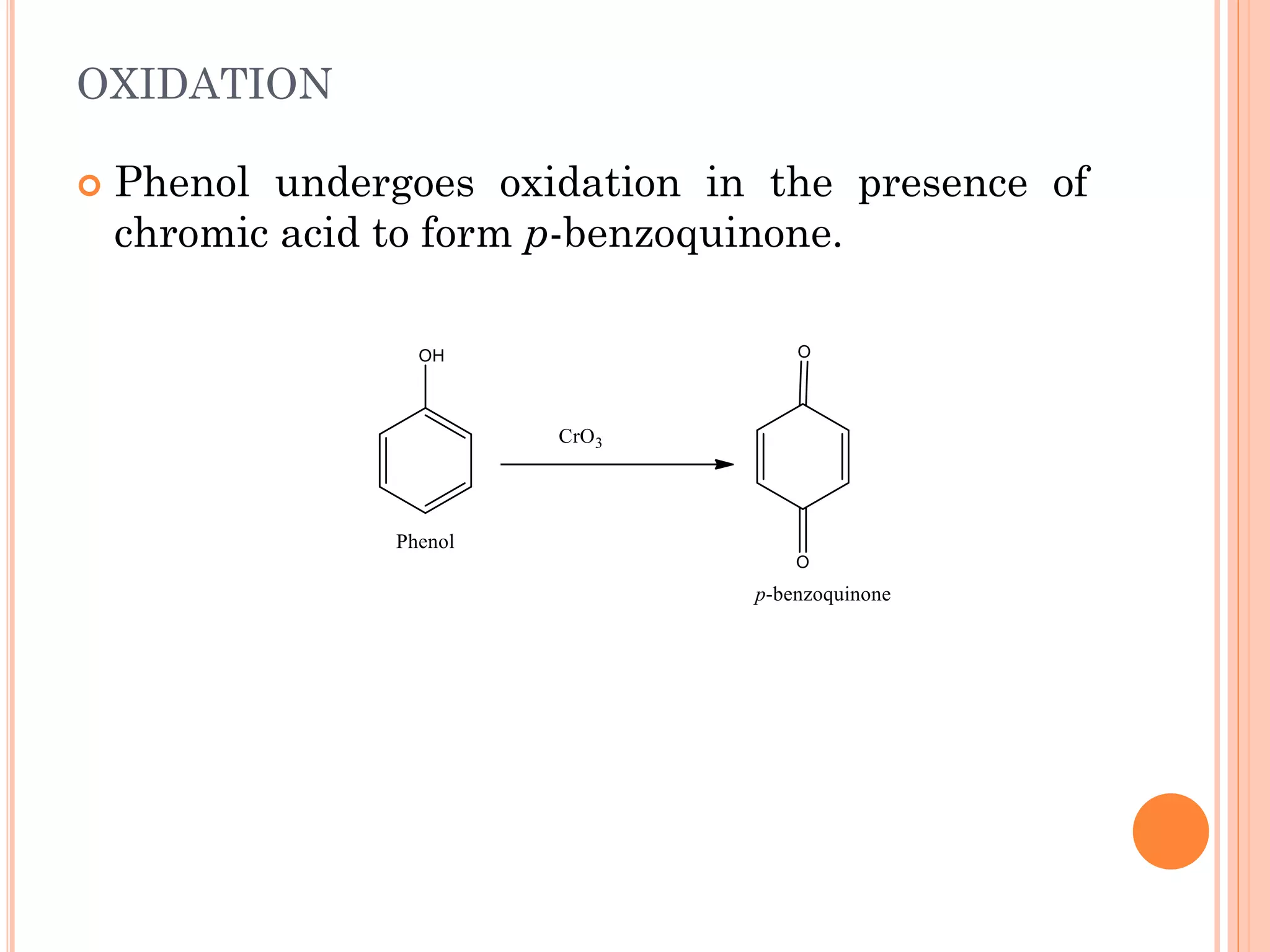
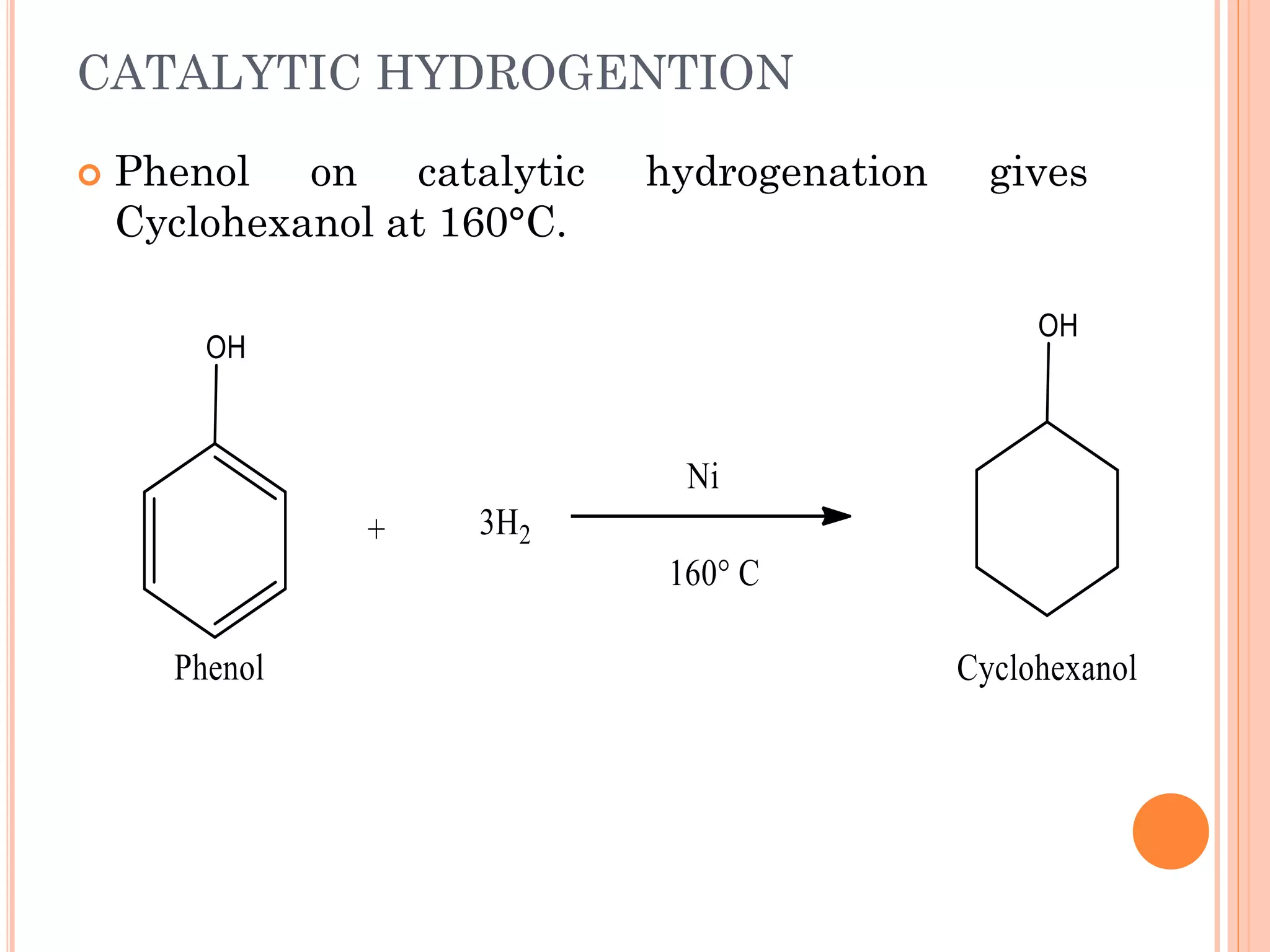
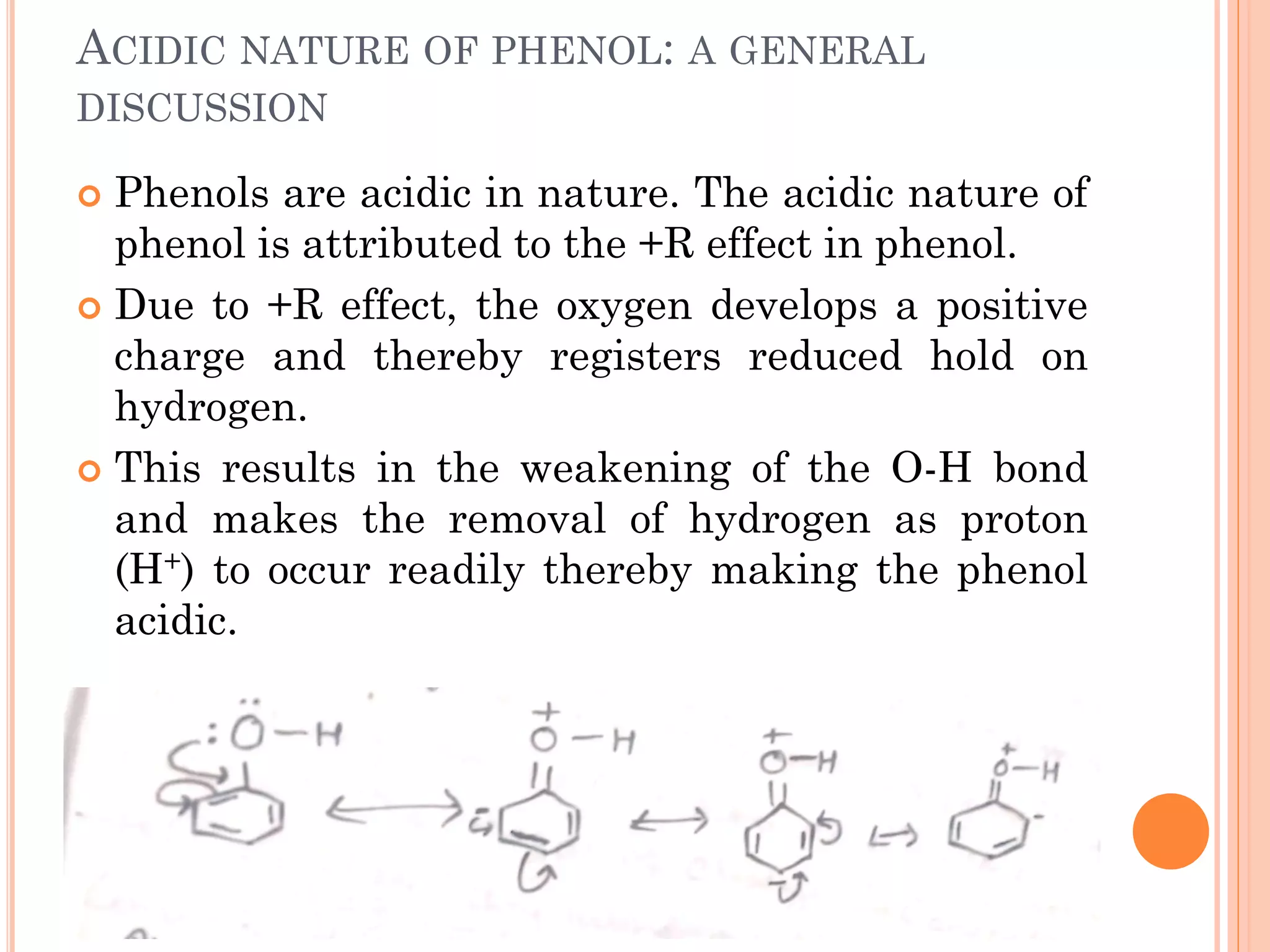
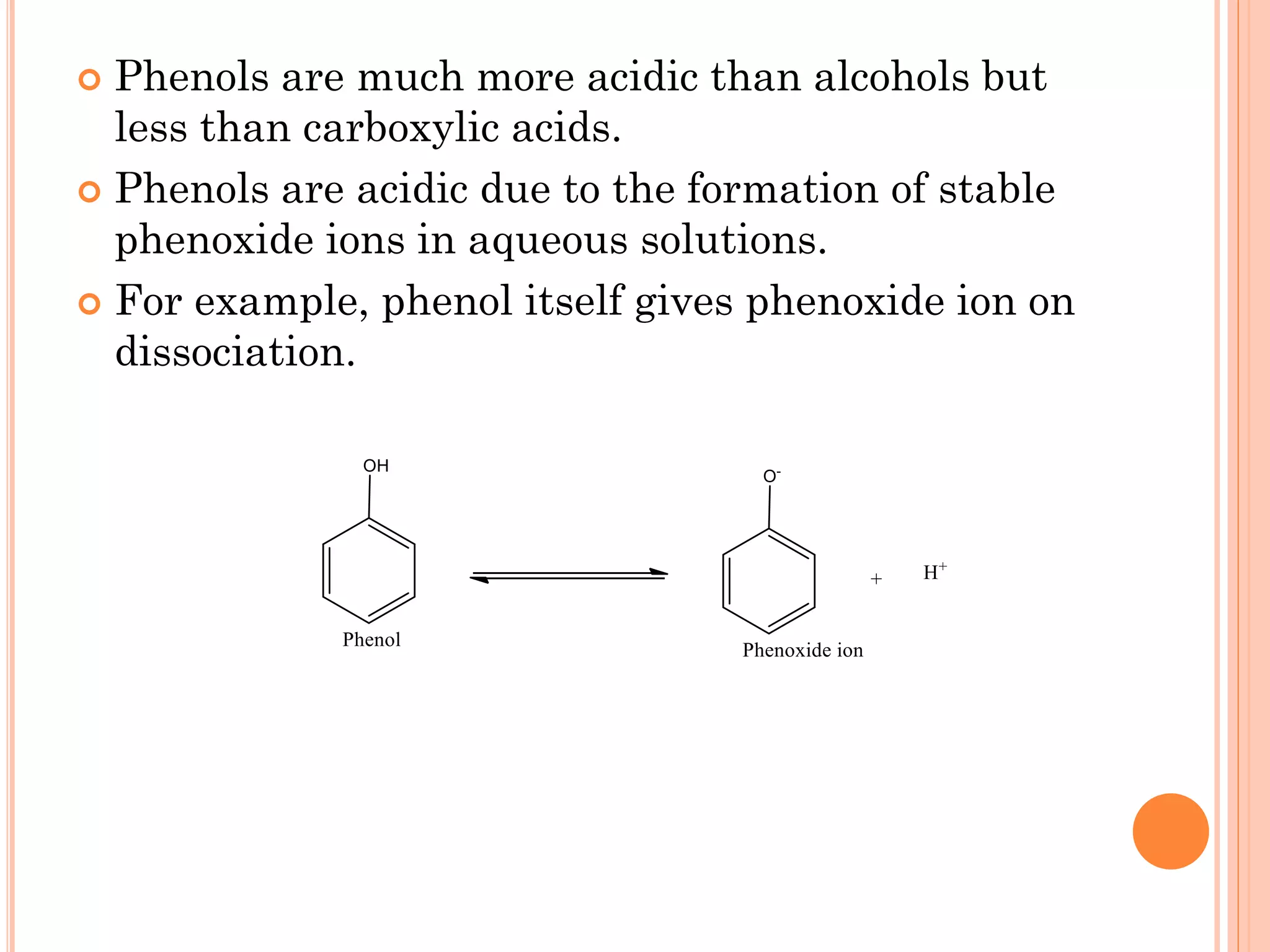
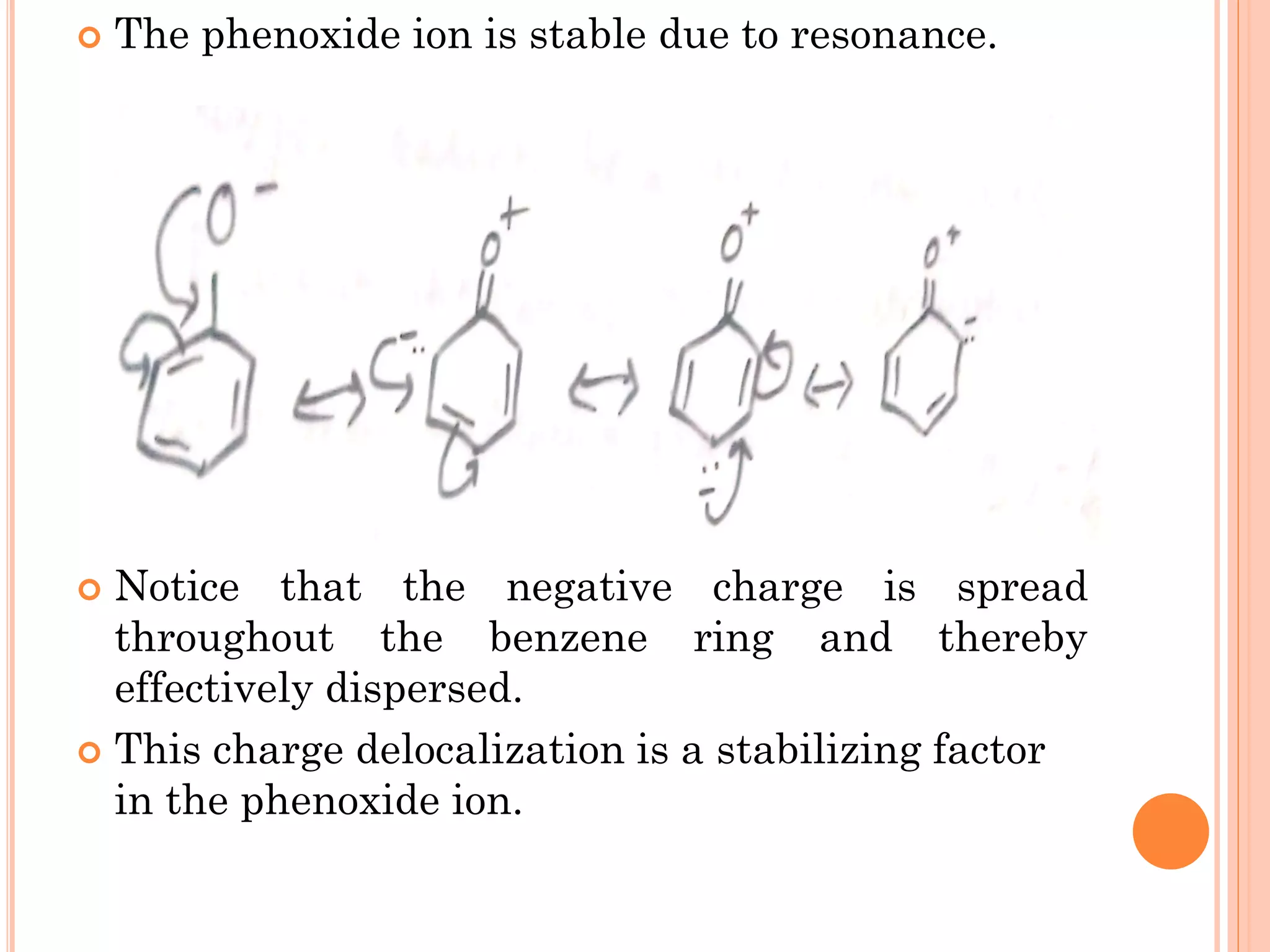
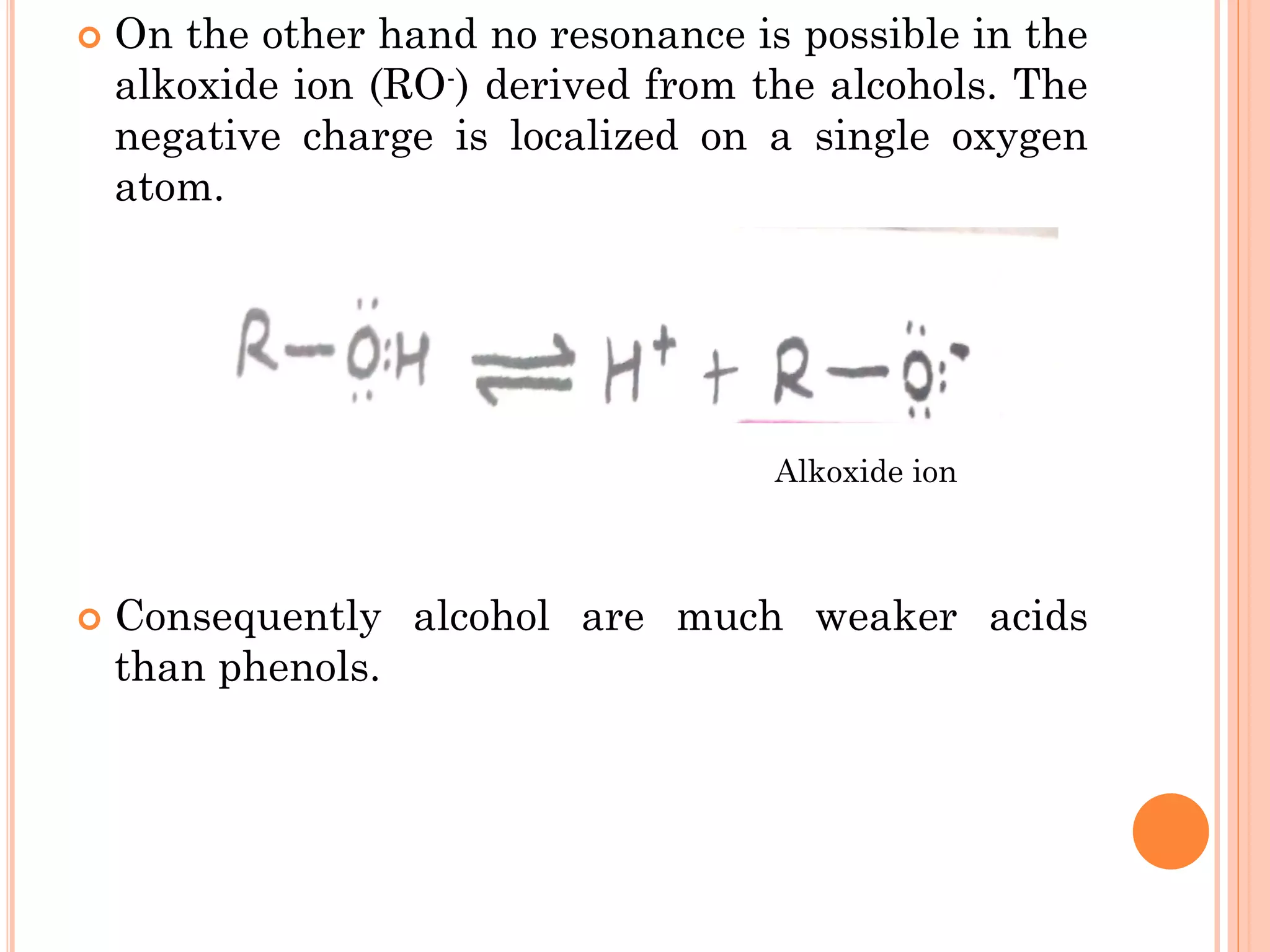
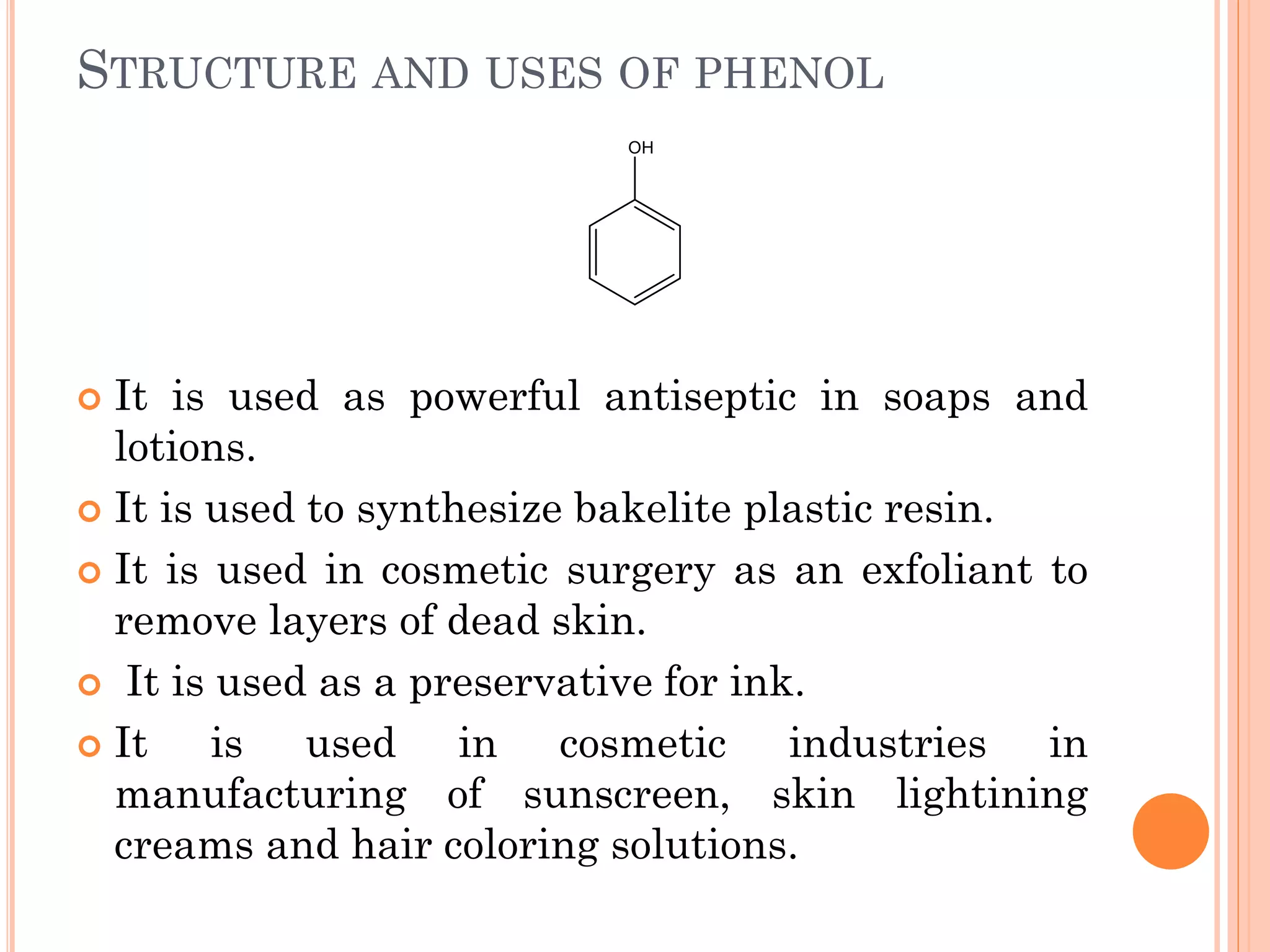
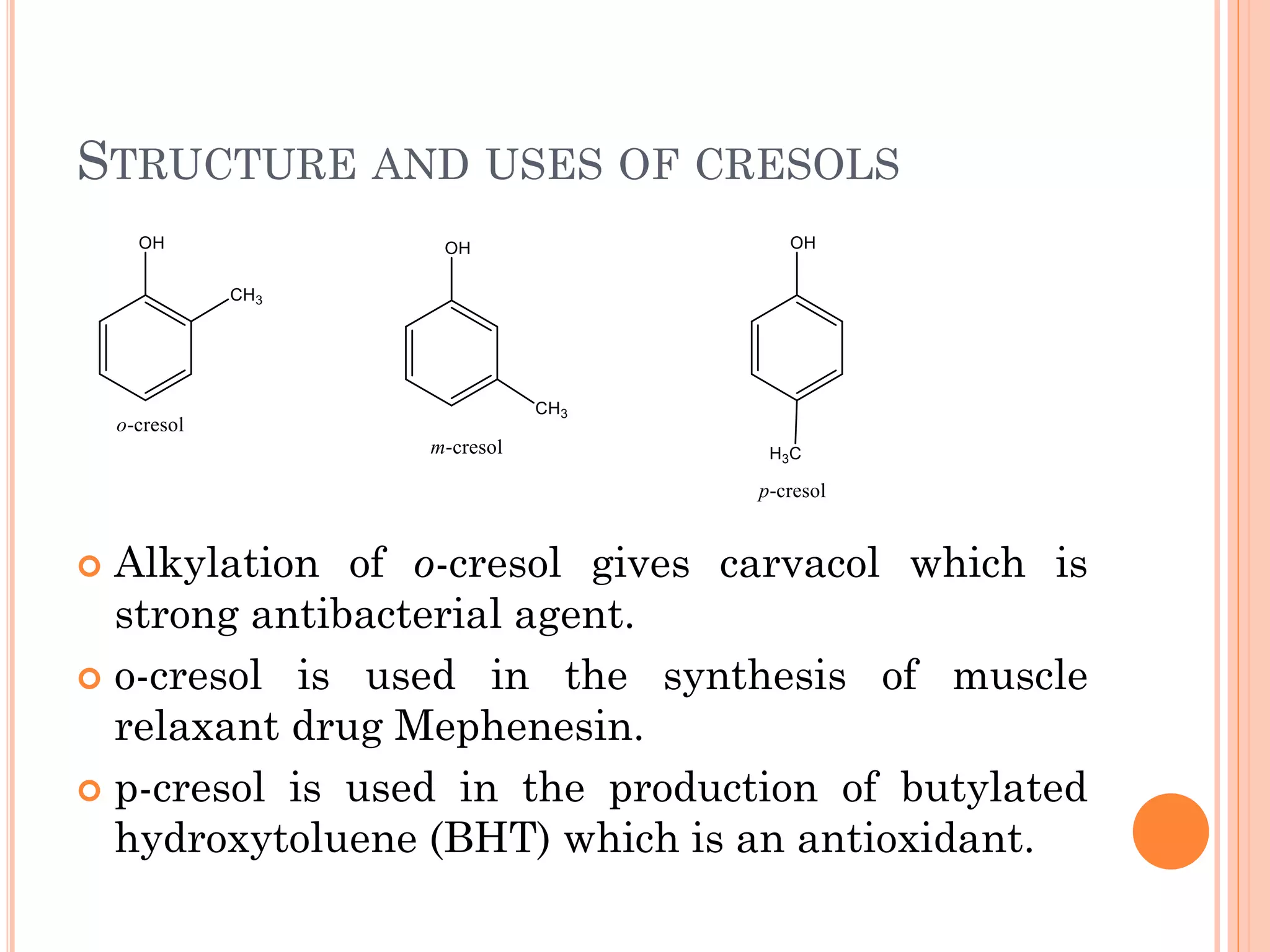
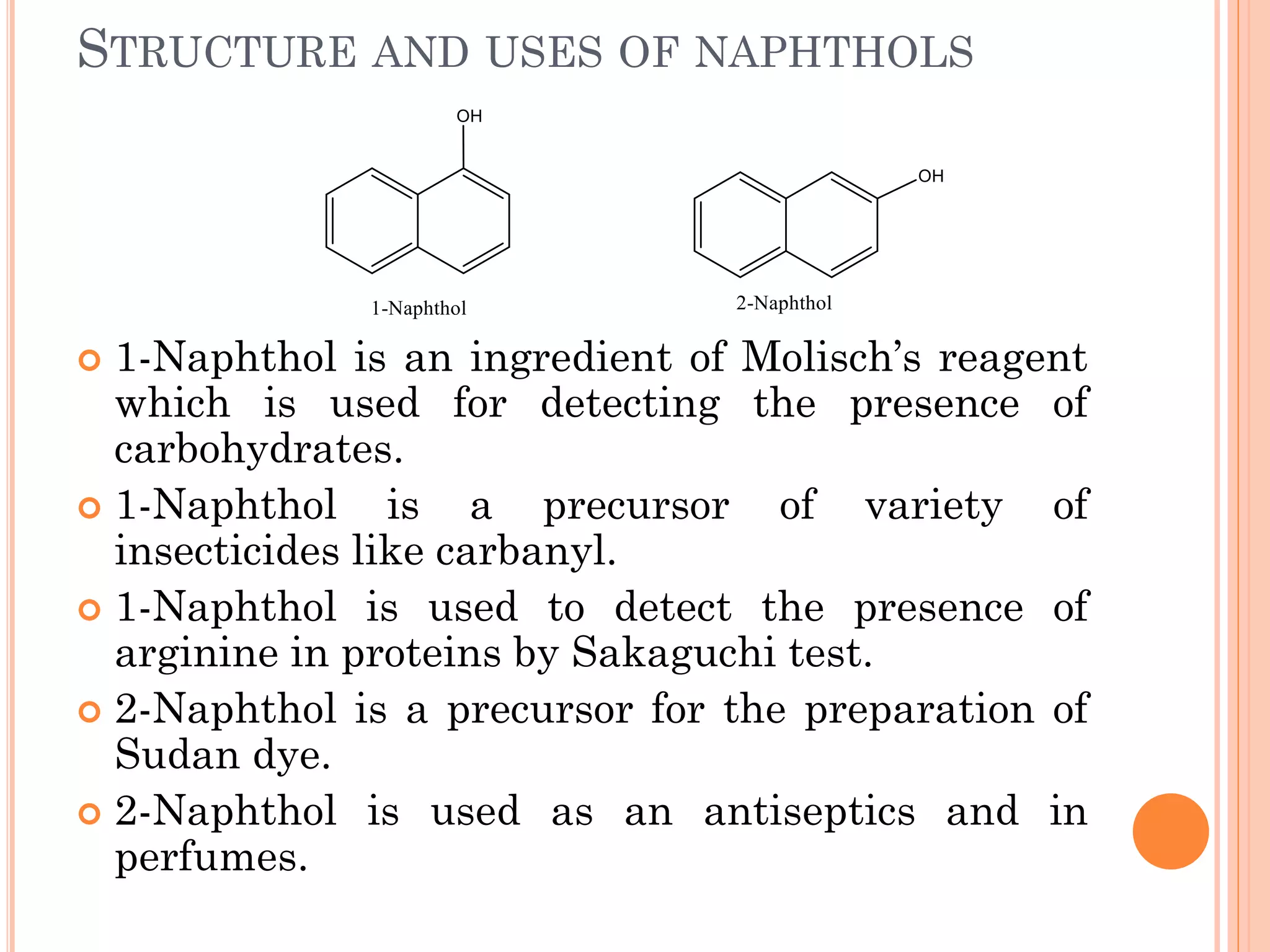
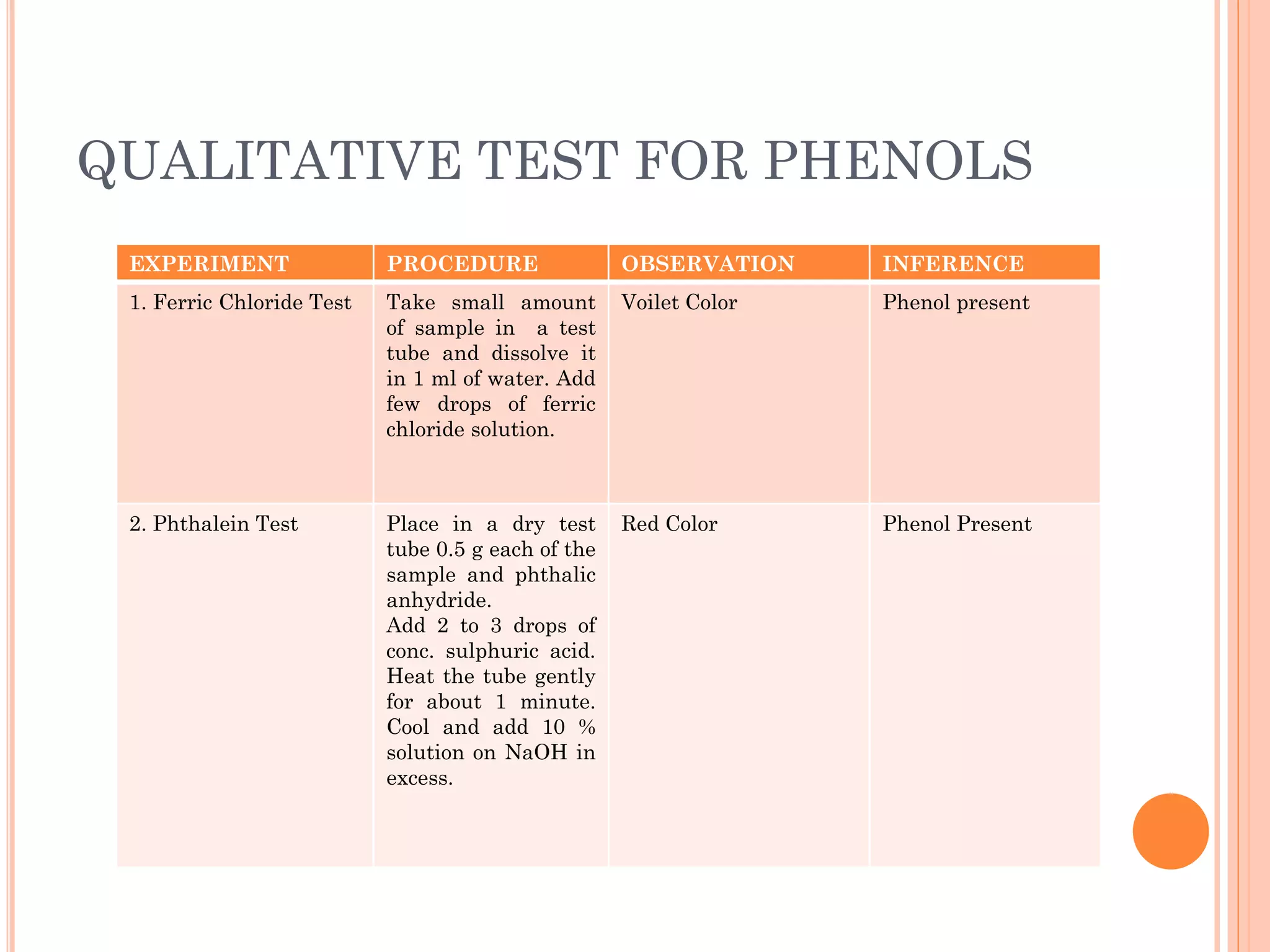
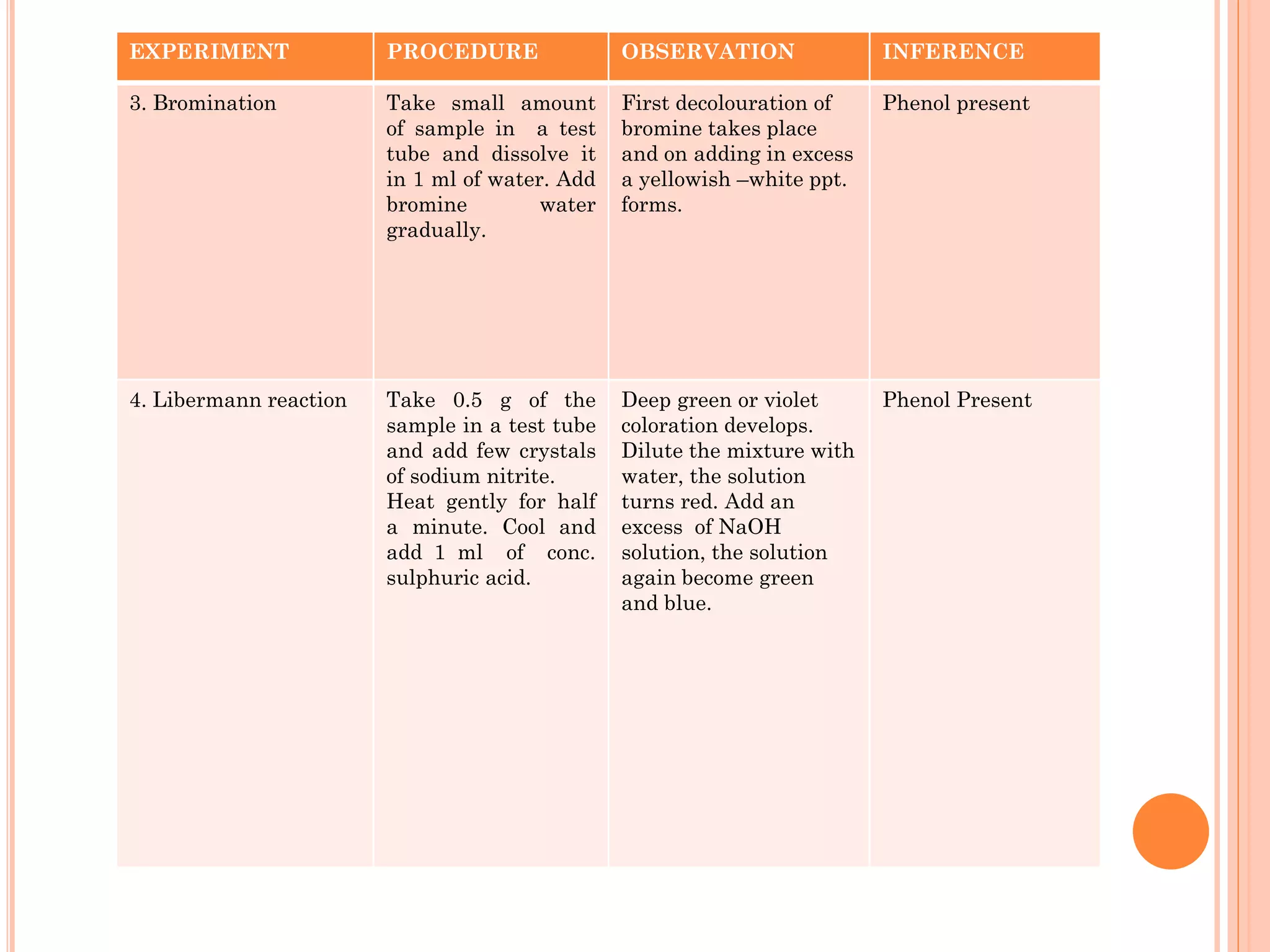
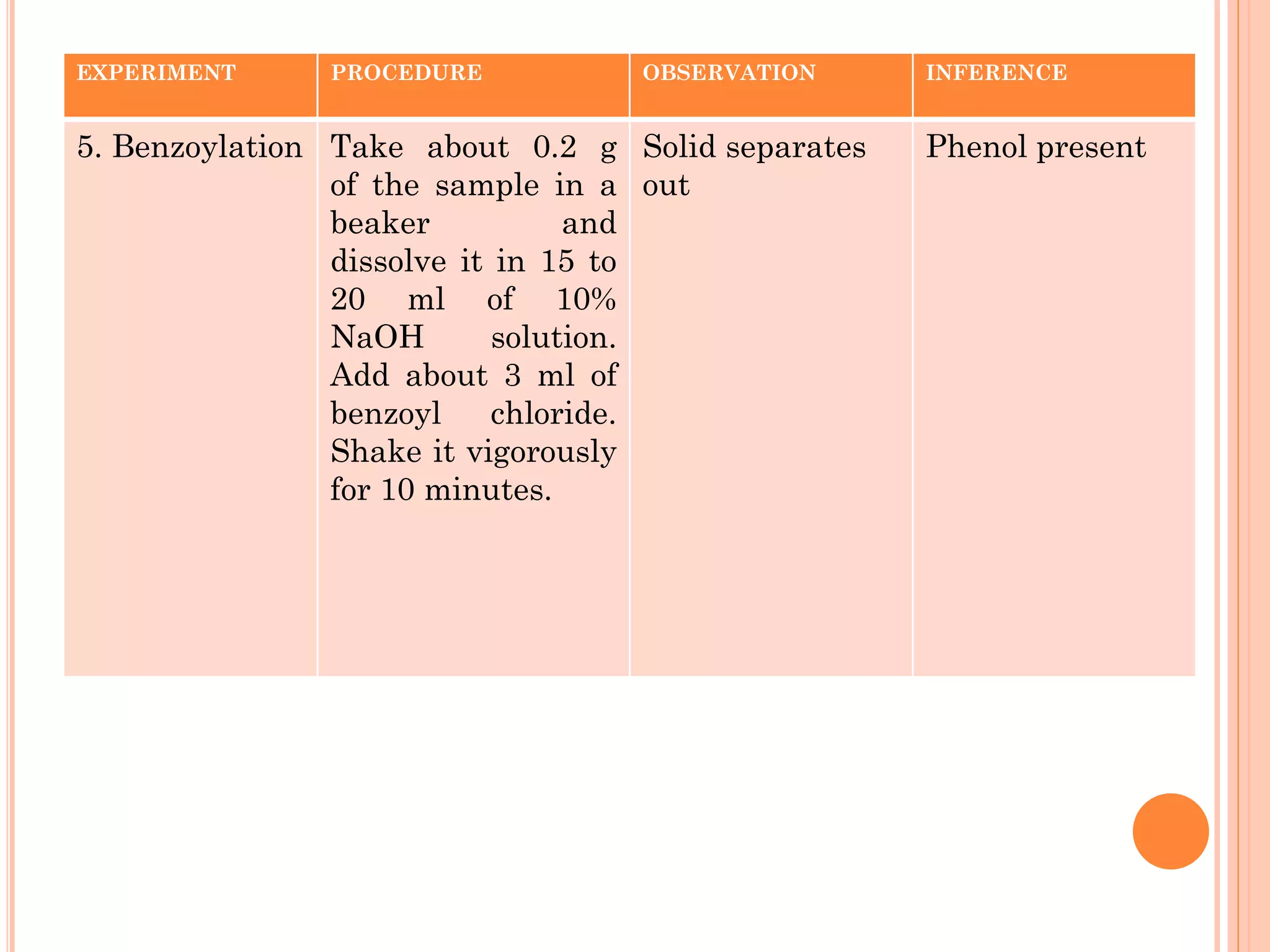
Phenols are organic compounds featuring hydroxyl groups attached to aromatic rings, with phenol (C6H5OH) as the simplest member. Their production methods include Dow's process, oxidation of cumene, and reactions with halides and acids, with various chemical properties such as acidity and reactivity described. Applications of phenols encompass use as antiseptics, in plastics like Bakelite, and in cosmetic products, with derivatives like cresols and naphthols also having significant industrial and pharmaceutical uses.