Embed presentation
Downloaded 50 times

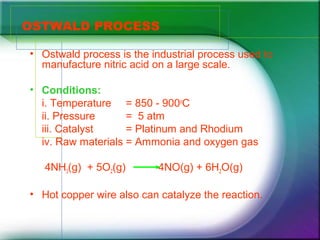
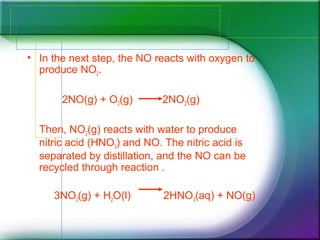




Nitric acid is manufactured industrially using the Ostwald process. The process involves reacting ammonia and oxygen gas over a platinum or rhodium catalyst at high temperatures and pressures to produce nitric oxide. The nitric oxide further reacts with oxygen to form nitrogen dioxide, which then reacts with water to produce nitric acid and nitric oxide in a cycle. The nitric acid is separated through distillation. One major use of nitric acid is in producing ammonium nitrate for nitrogen-based fertilizers.





