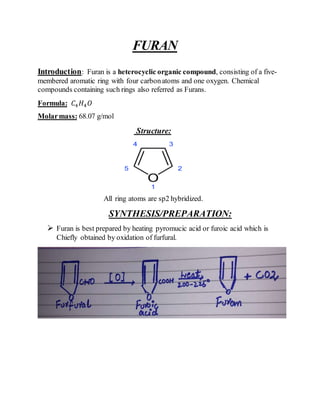
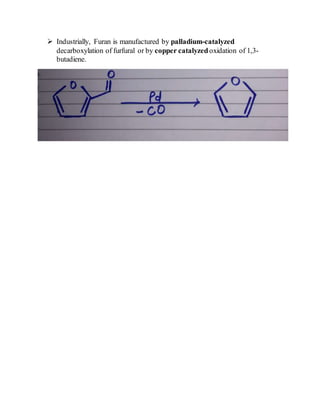
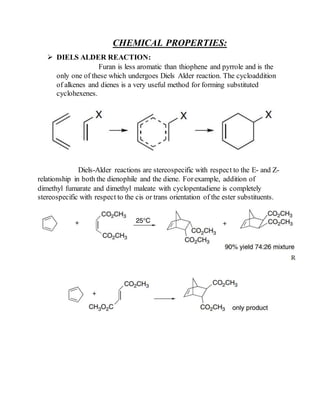
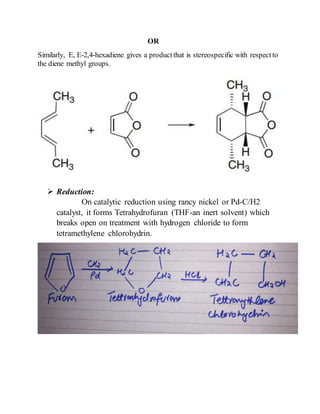
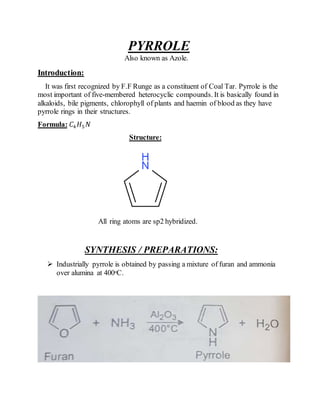
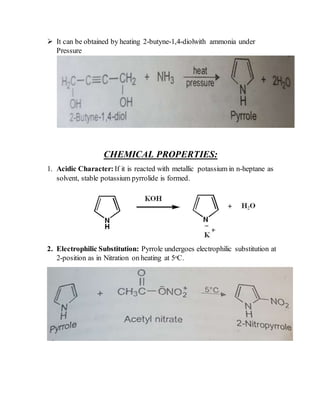
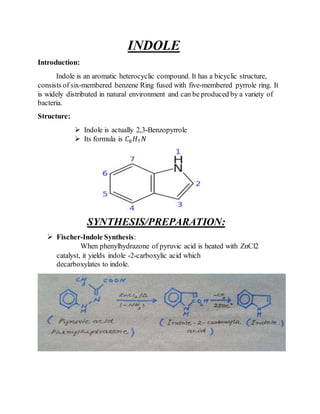
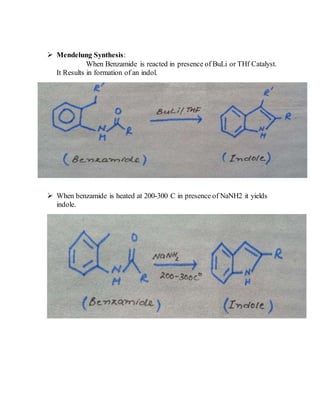
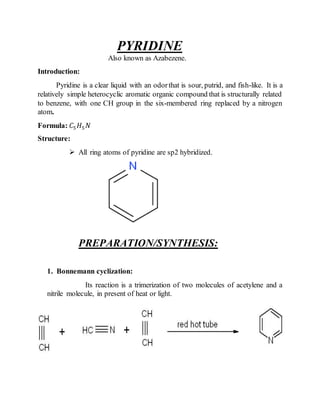
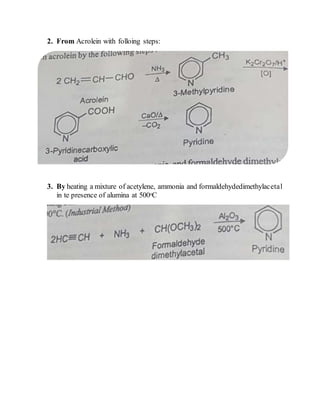
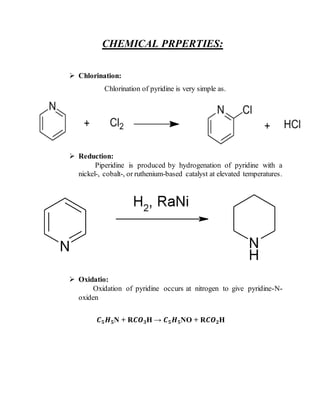
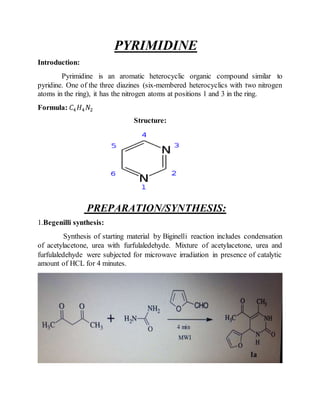
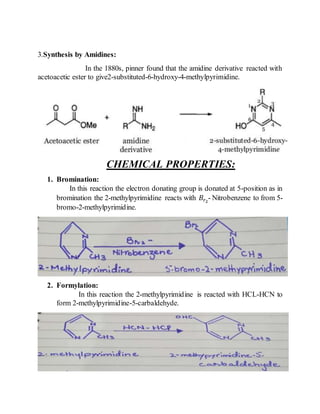
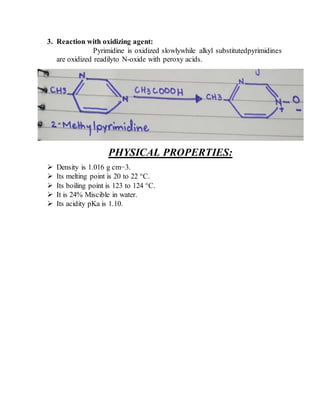
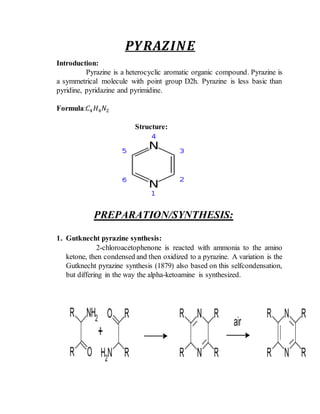
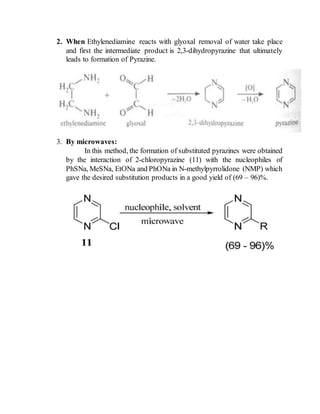
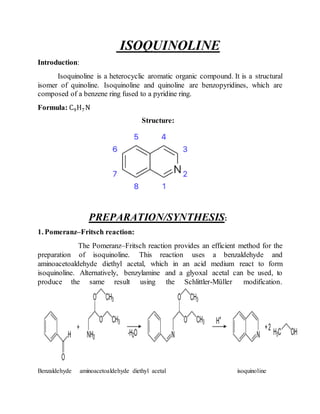
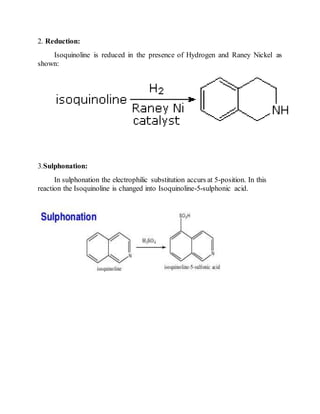
This document provides information on several heterocyclic organic compounds - furan, pyrrole, indole, thiophene, and pyridine. It discusses the structure, properties, synthesis, and applications of each compound. The key points are:
1) These compounds contain aromatic rings with at least one heteroatom such as oxygen, nitrogen, or sulfur. They are important in biomolecules, drugs, dyes, and other applications.
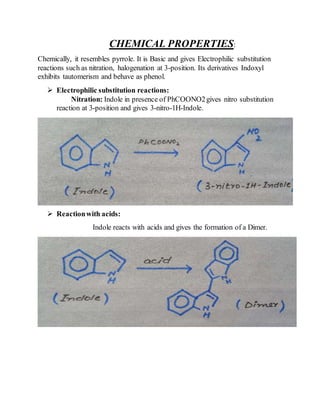
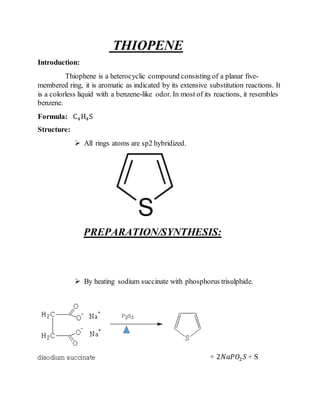
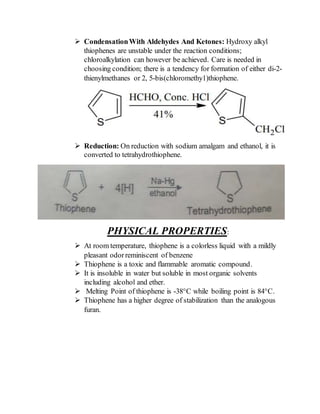
2) Their properties depend on the hybridization and reactivity of the ring atoms. Electrophilic aromatic substitution occurs commonly.
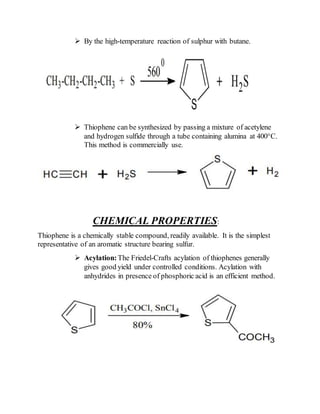
3) Synthesis methods include thermal decomposition, acid or base catalyzed reactions, and rearrangement reactions.
4)