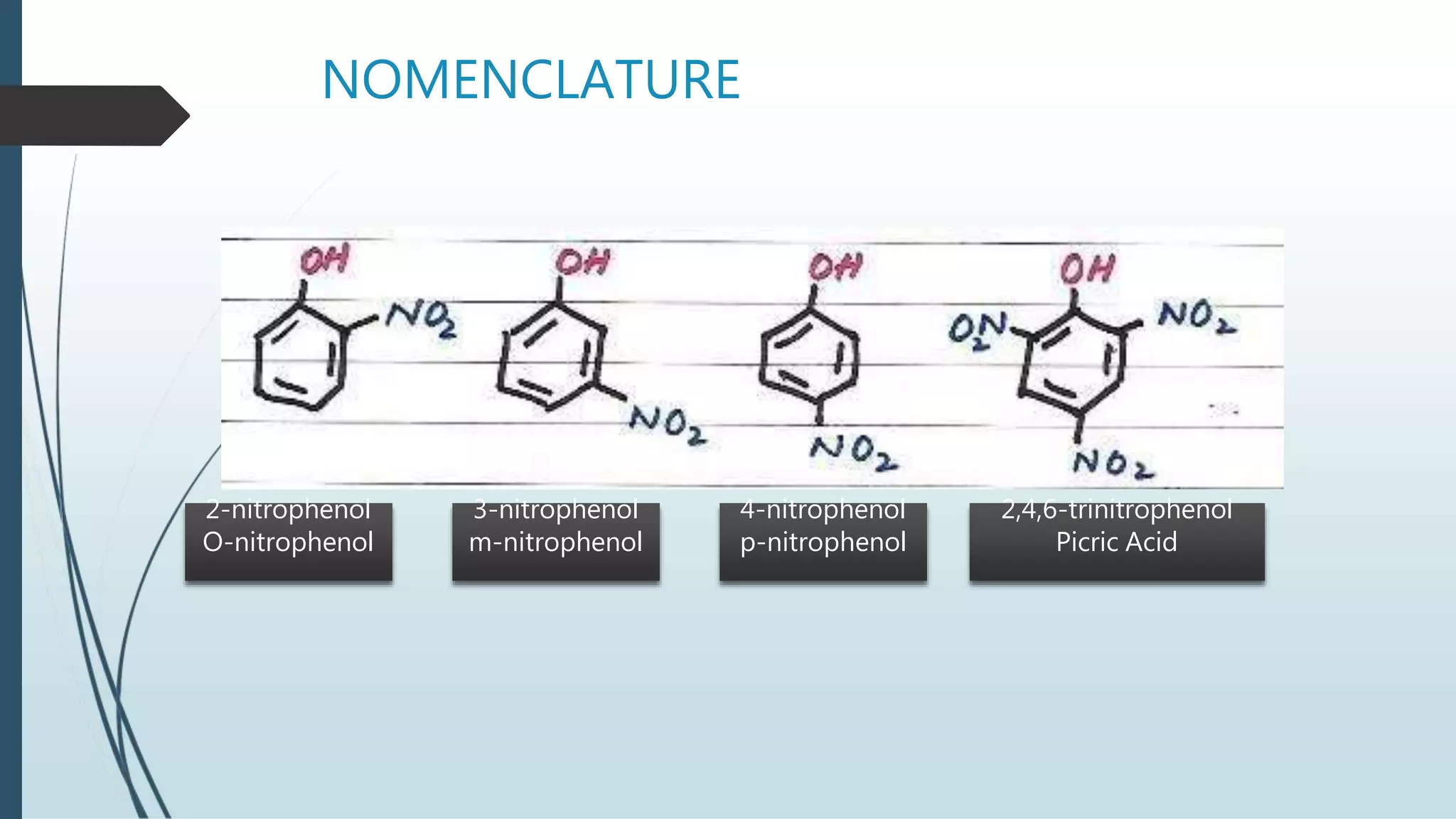
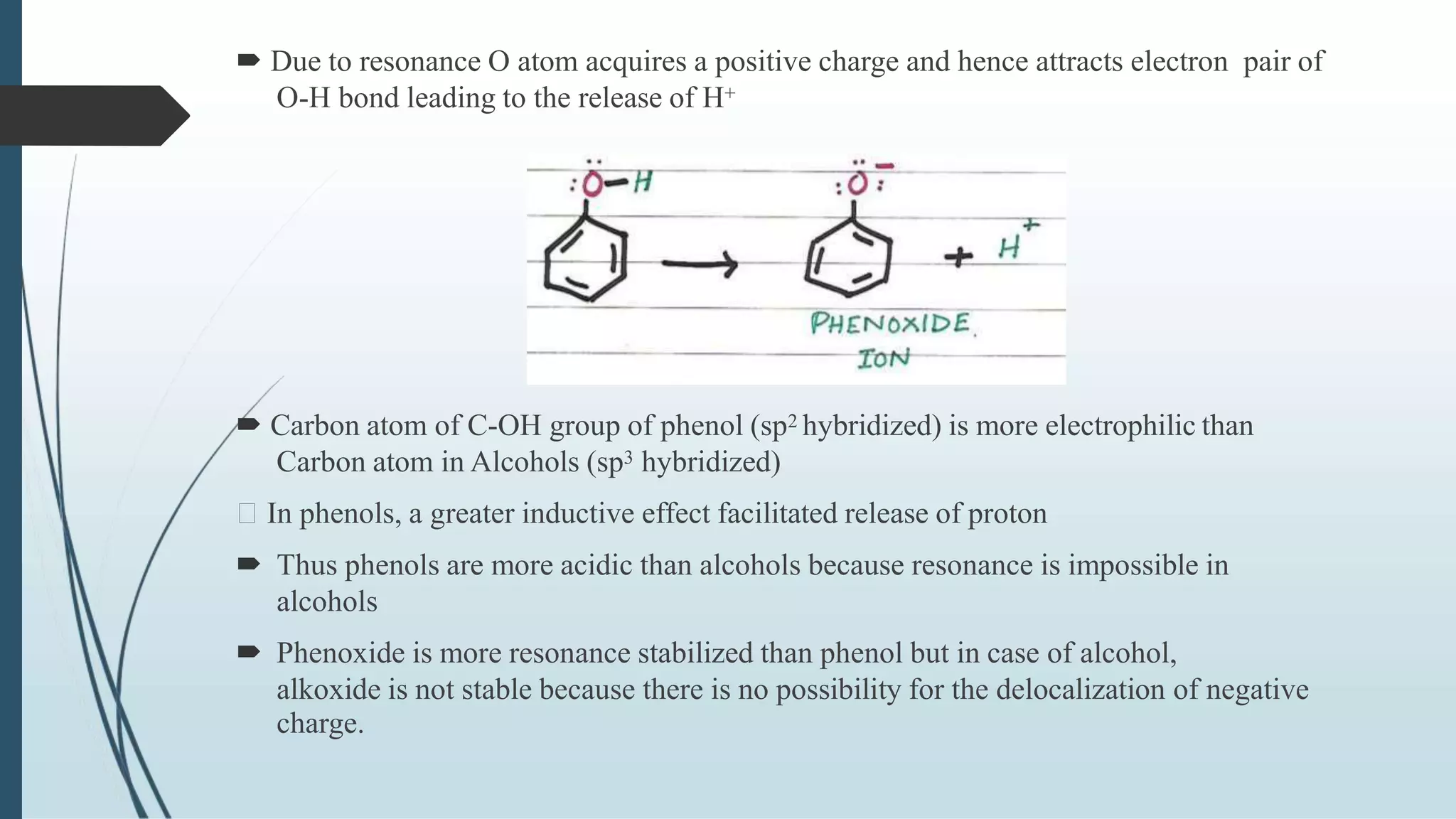
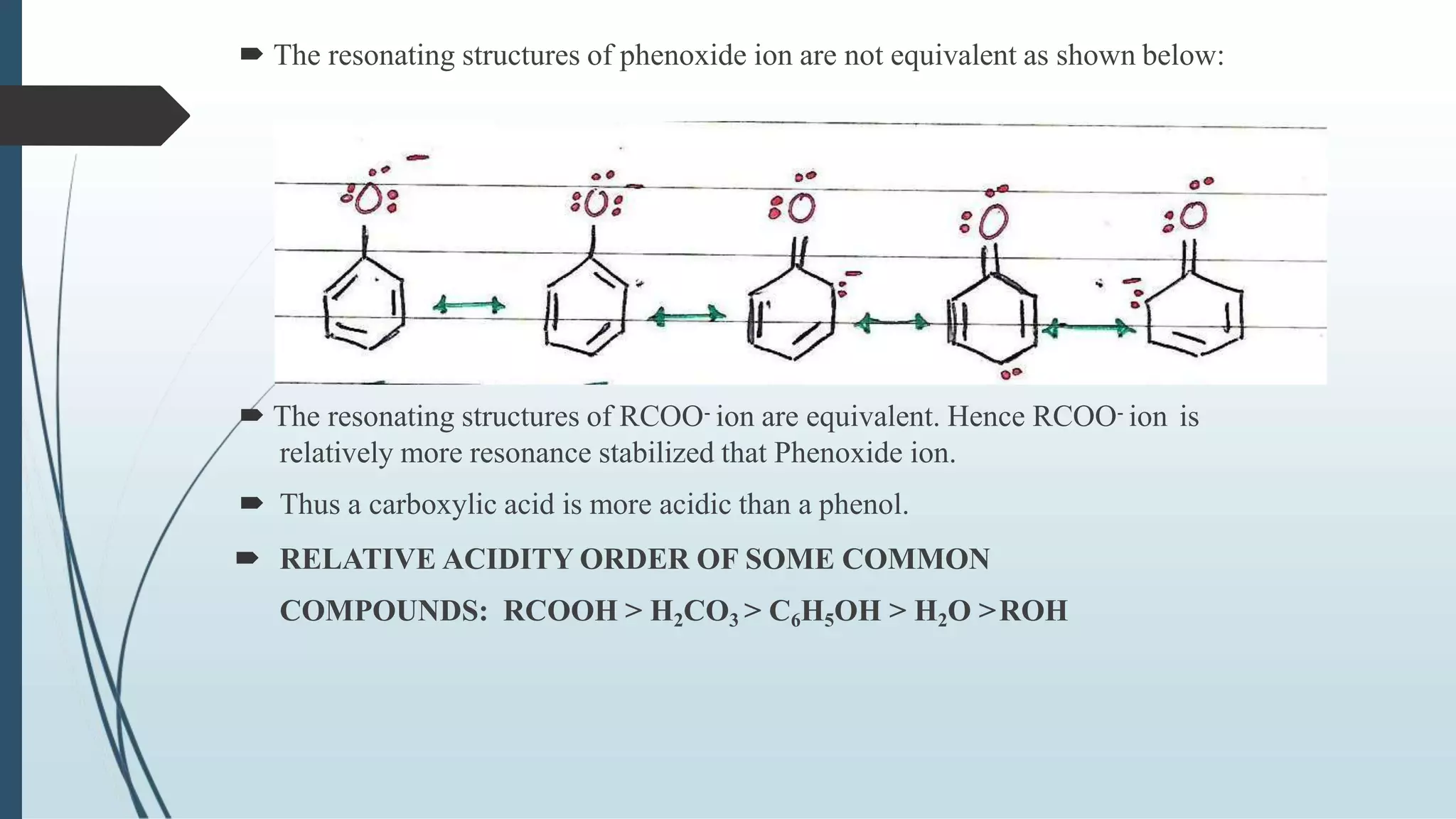
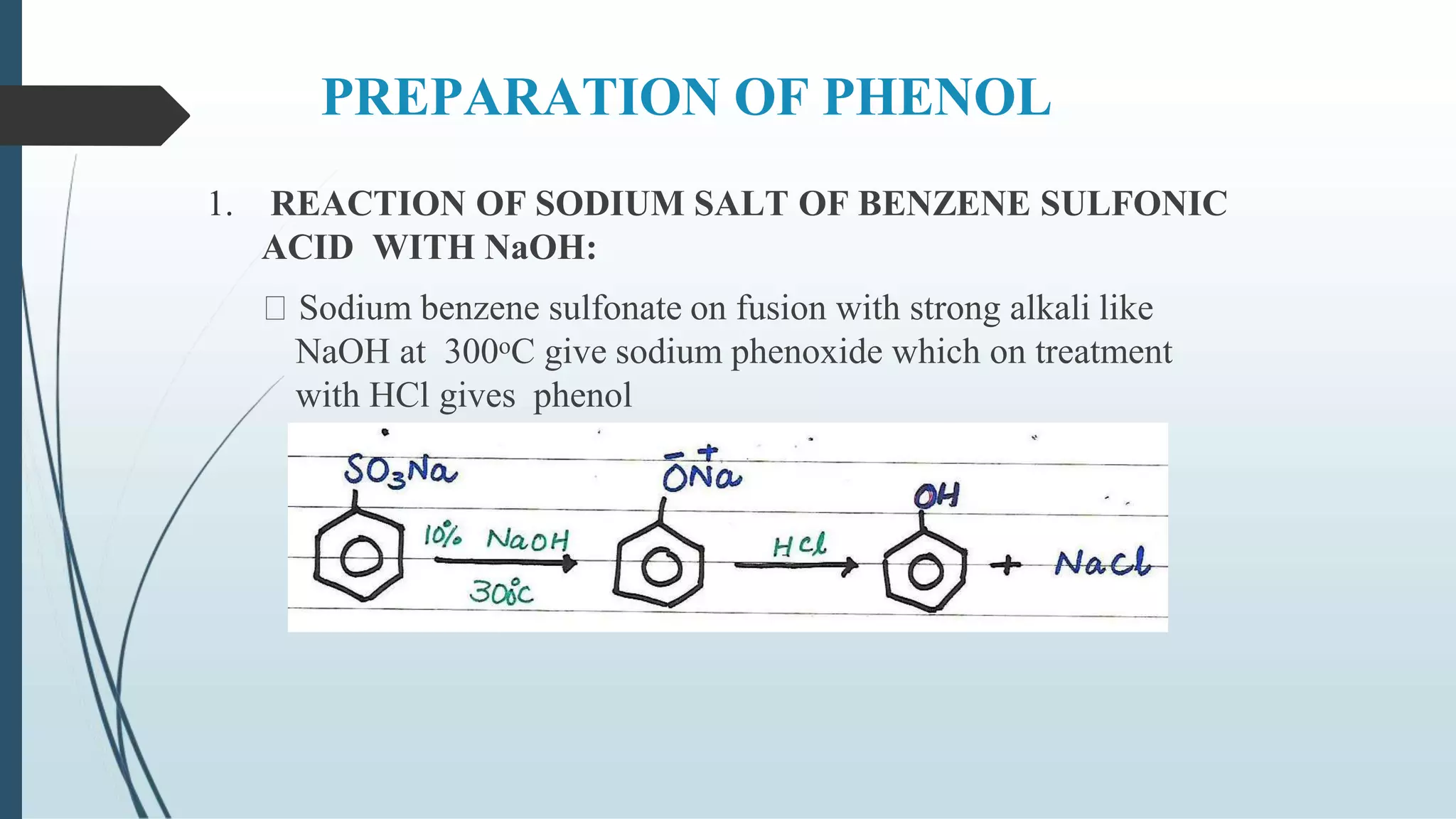
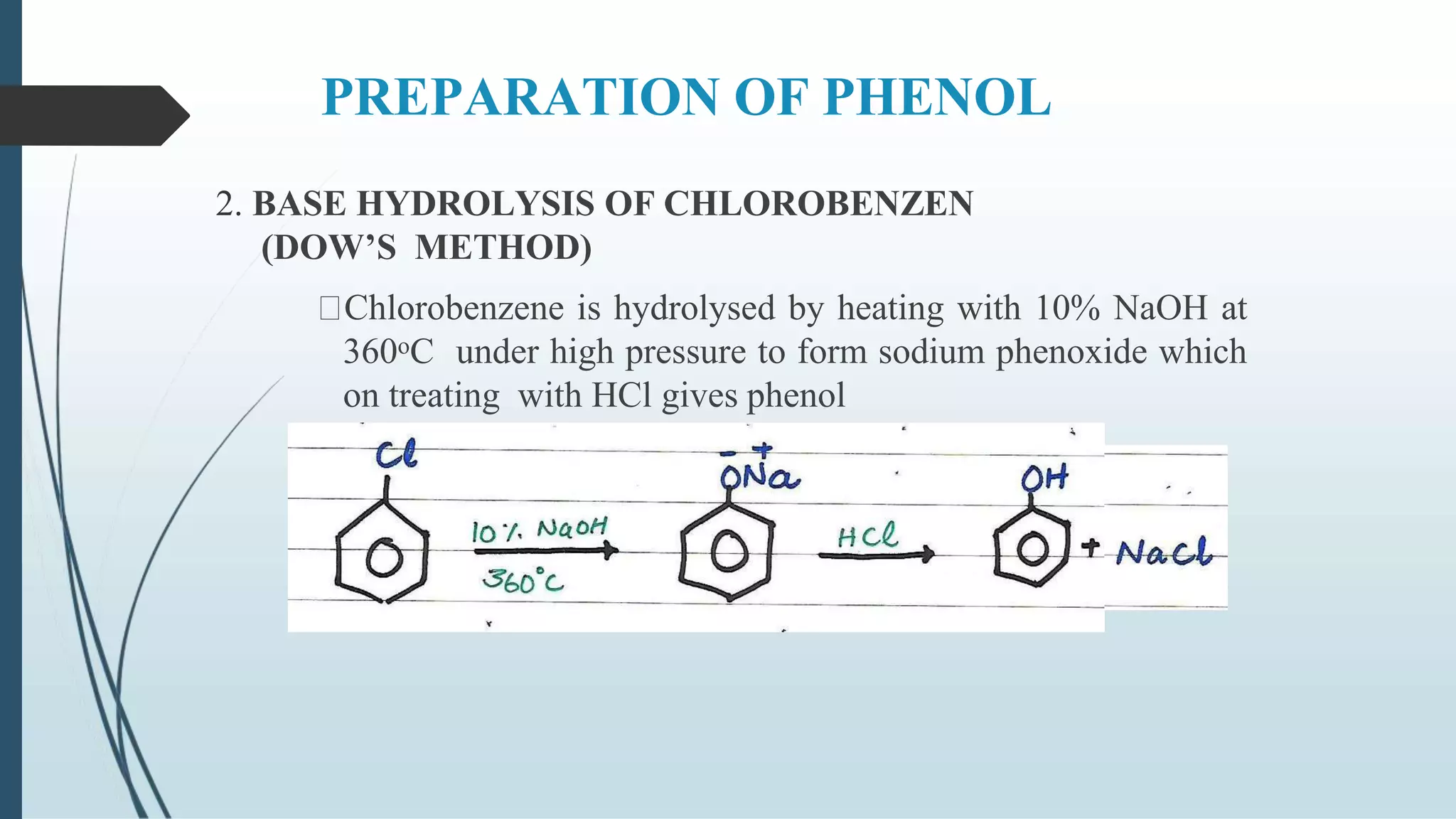
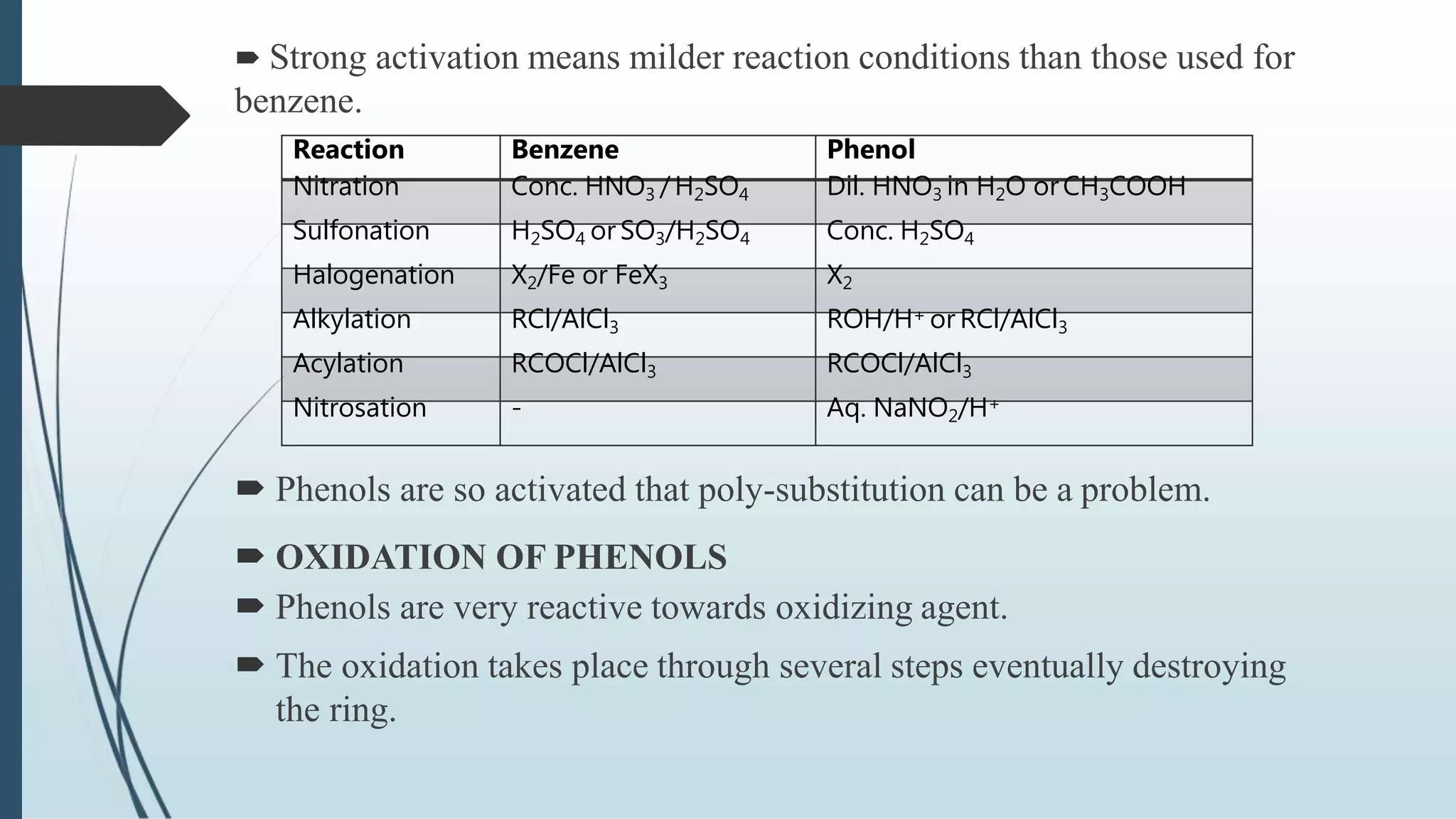
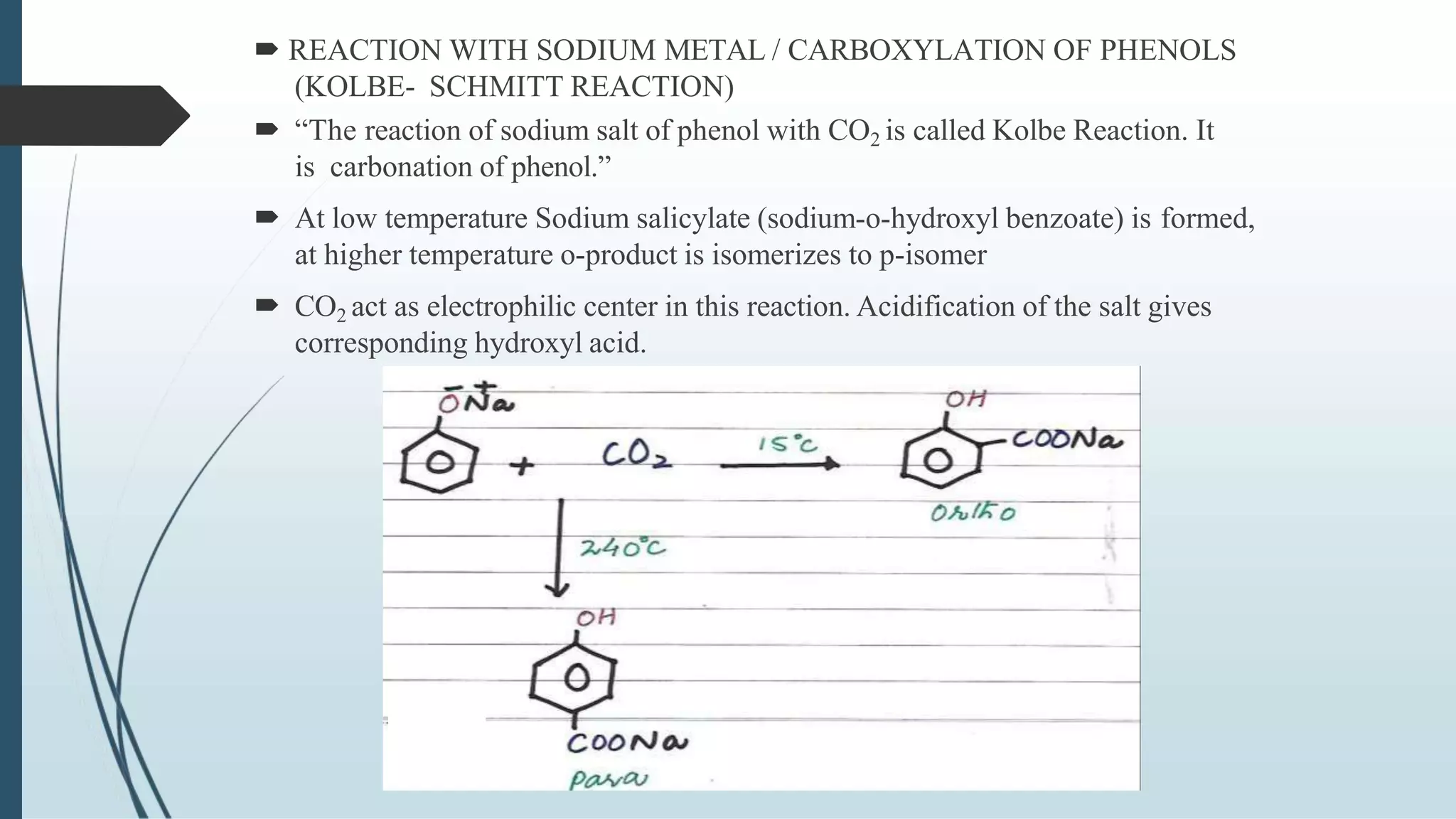
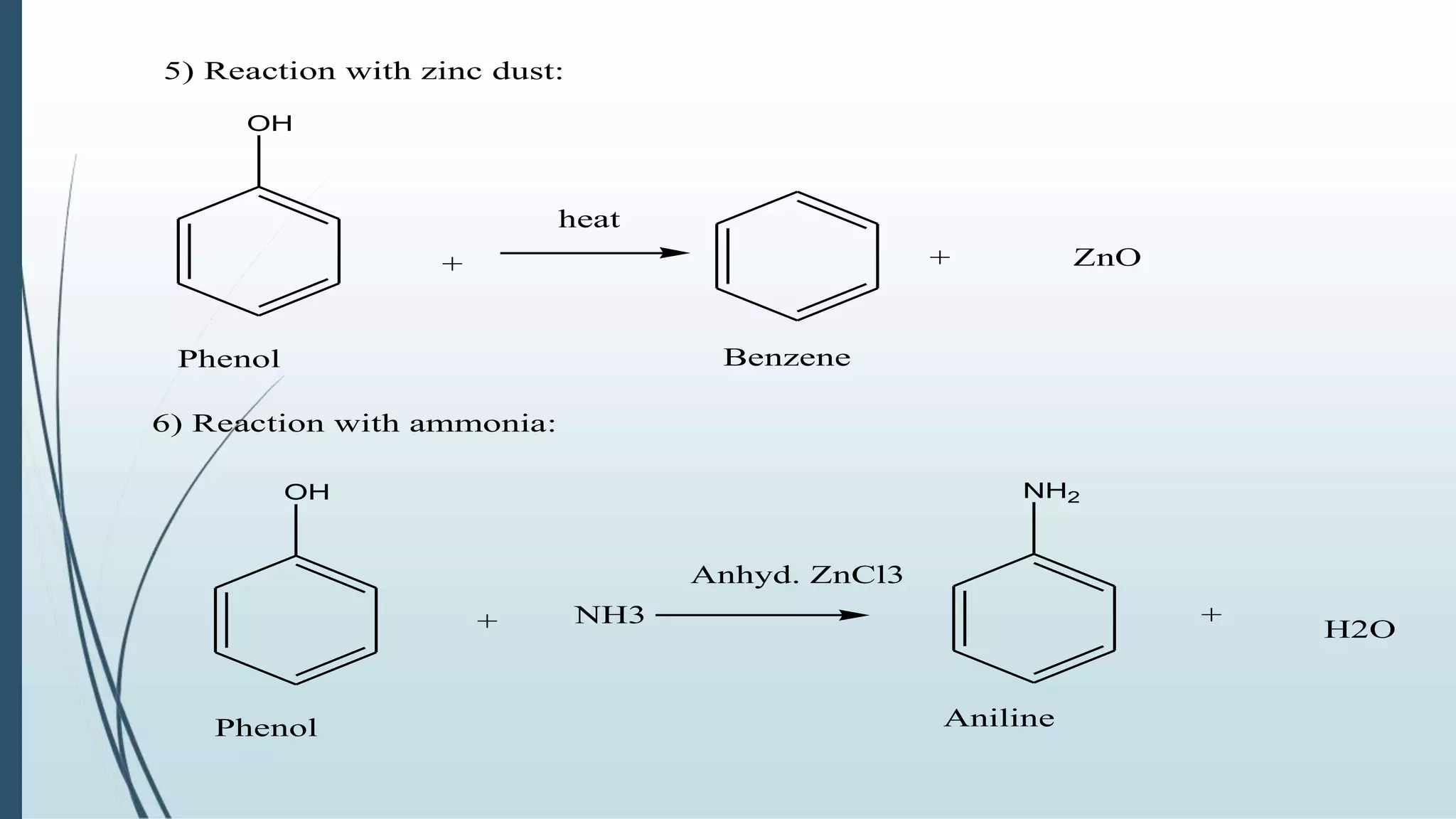
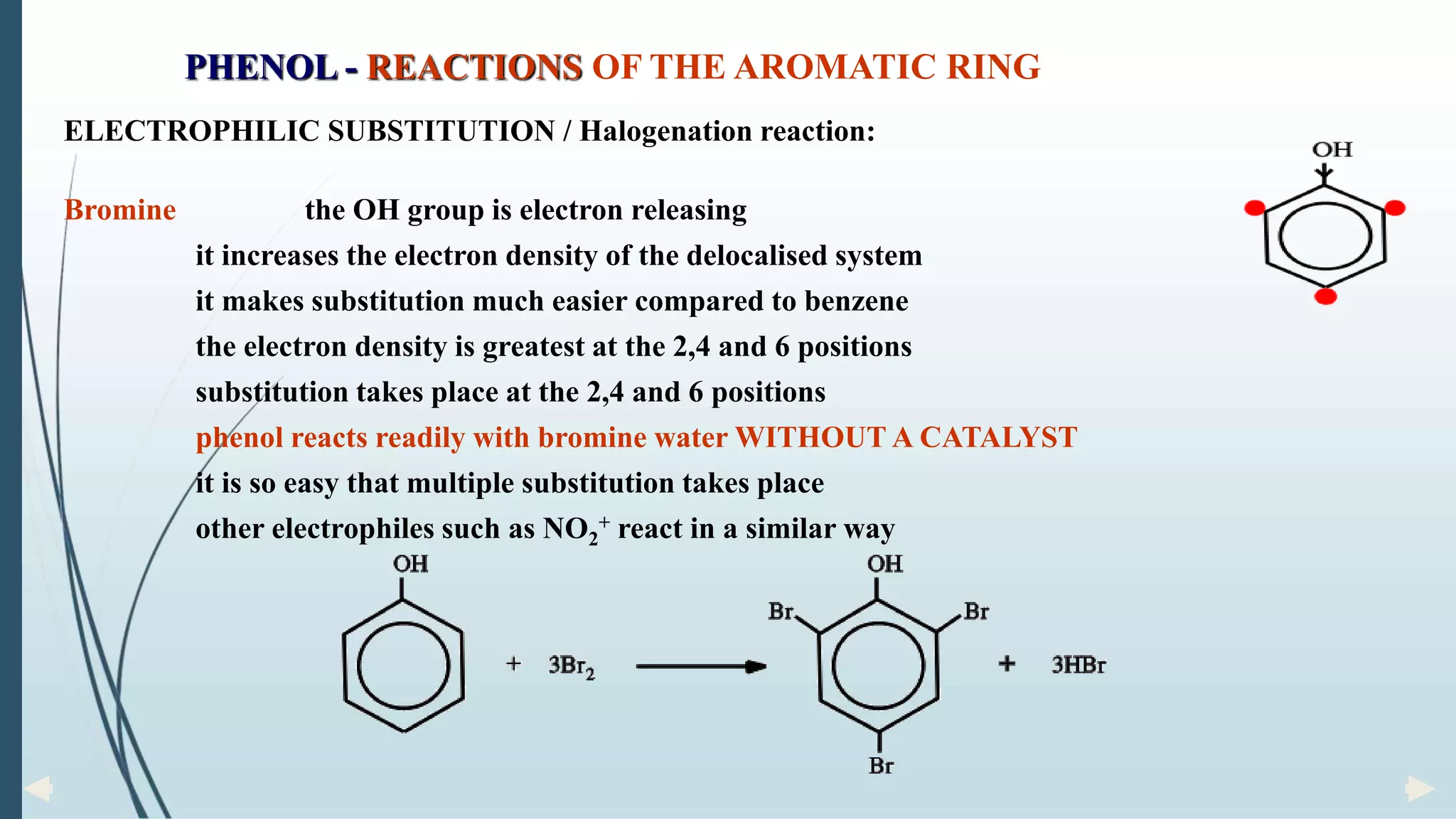
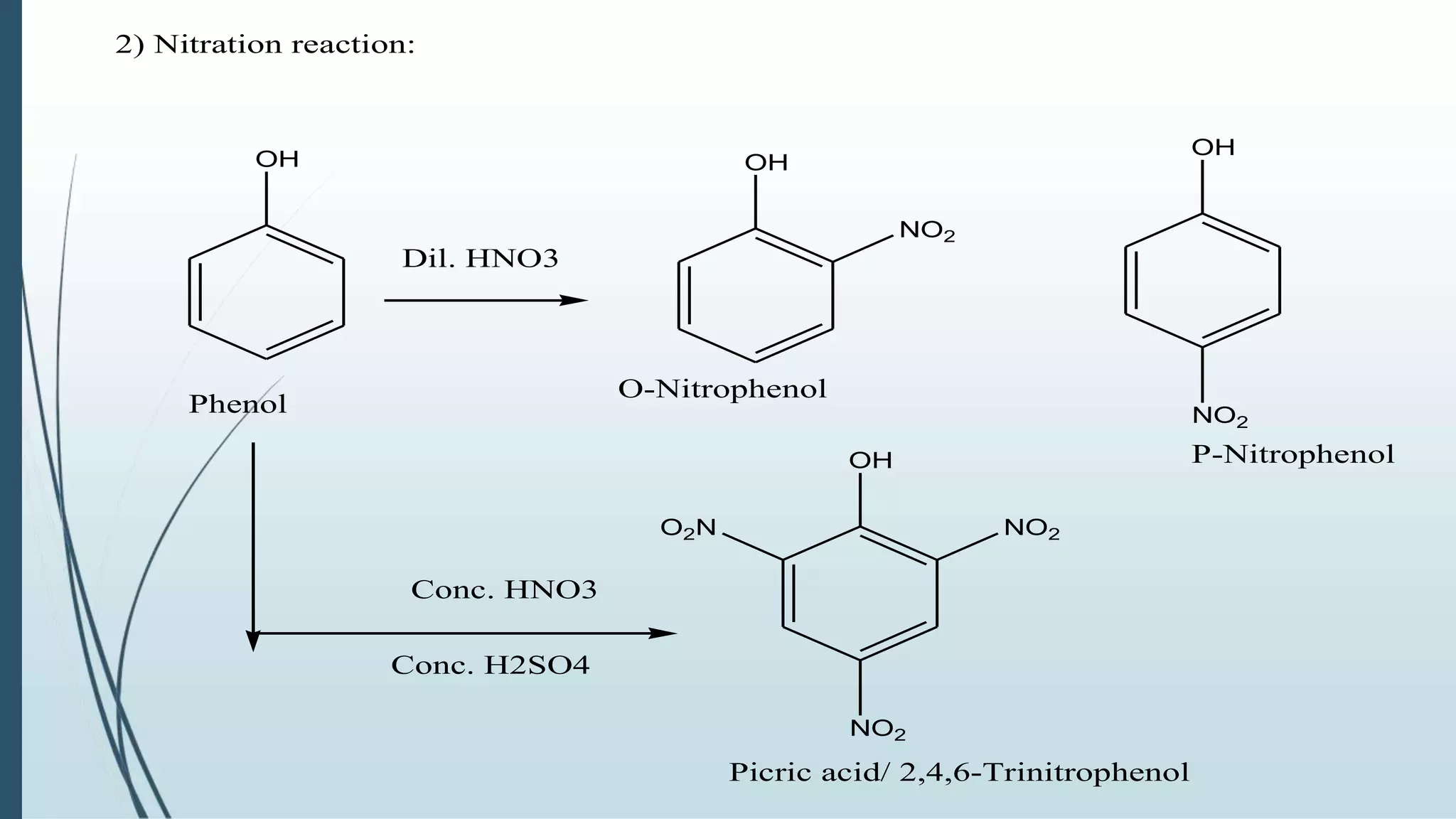
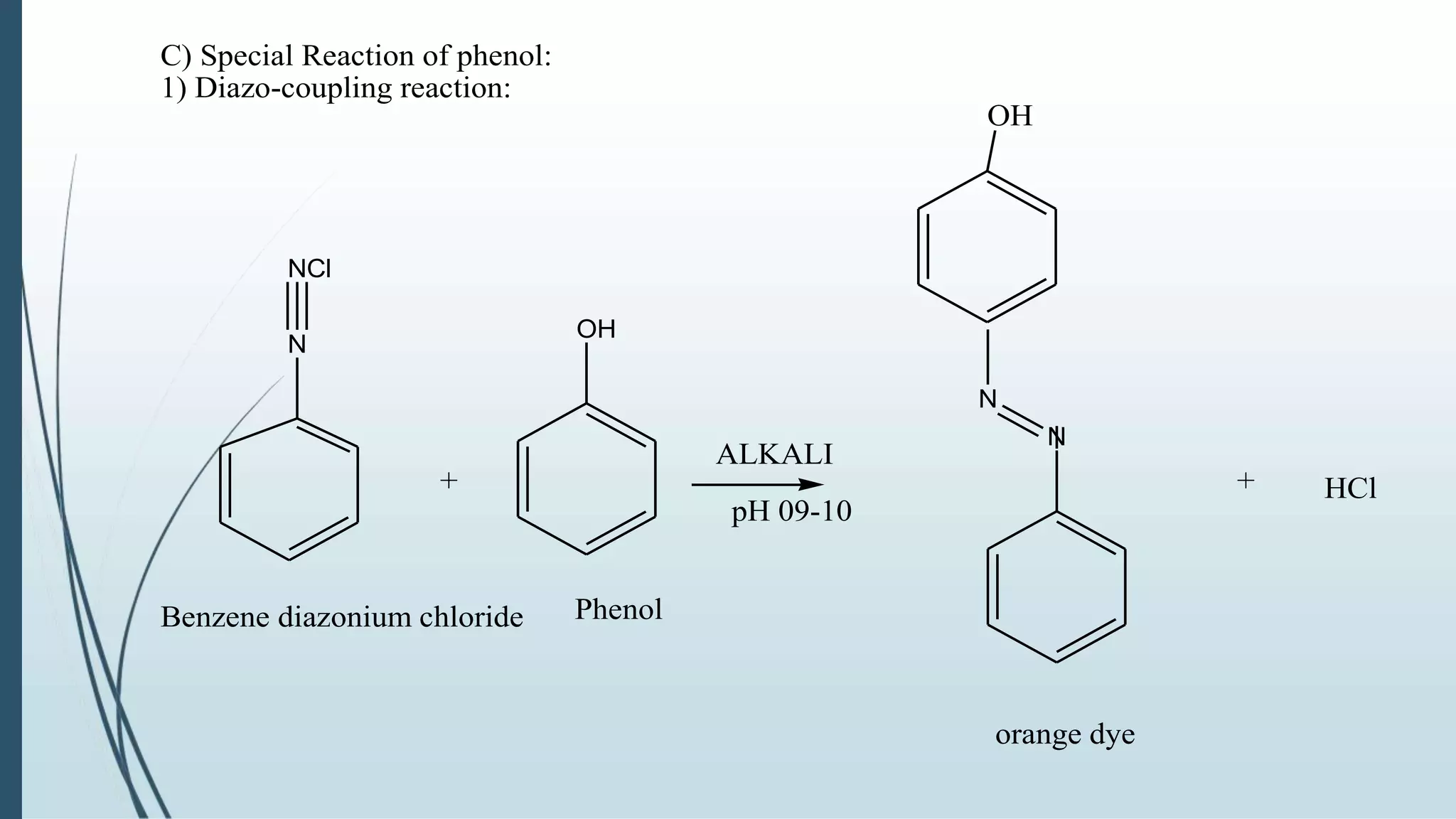
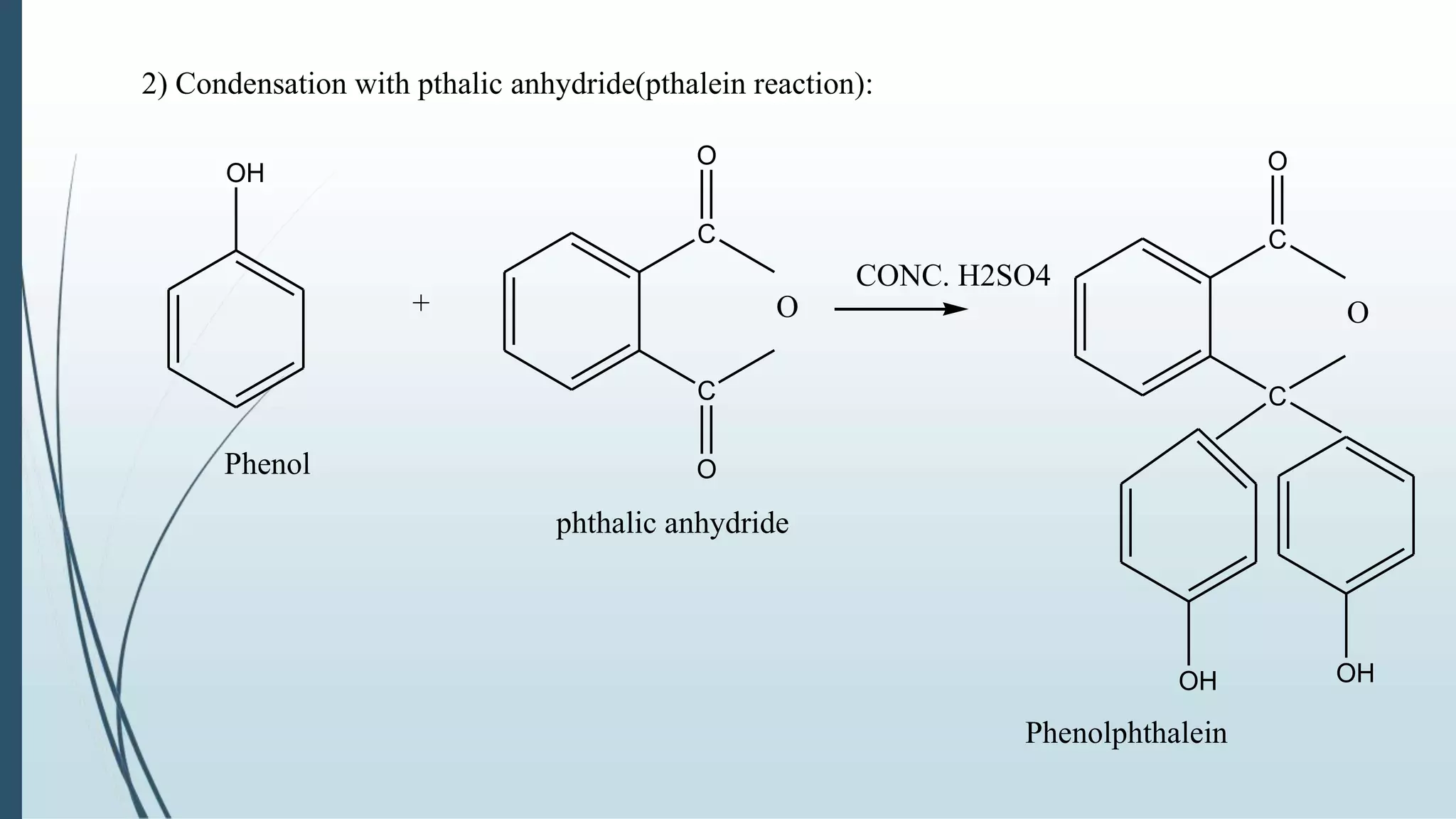
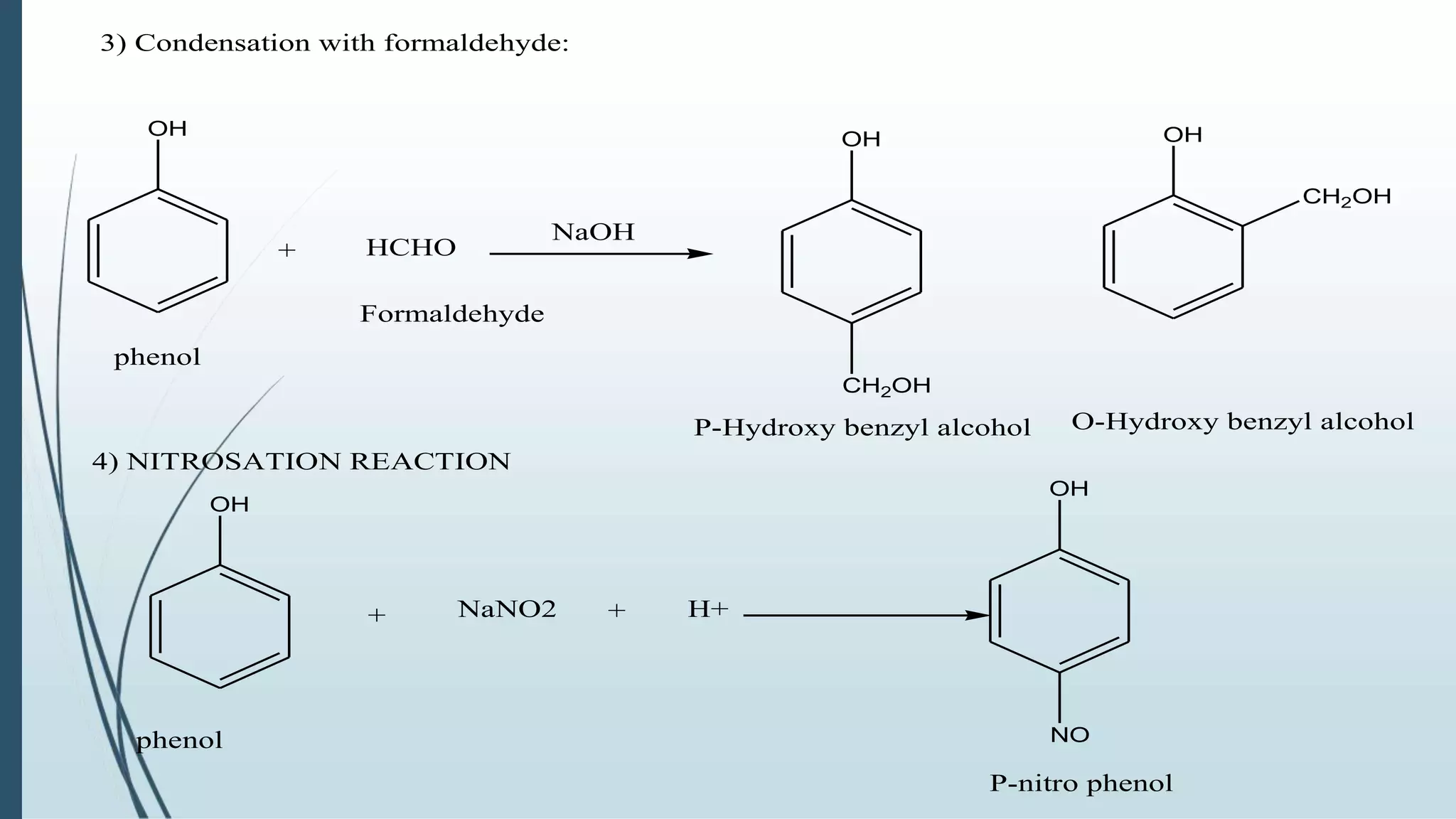
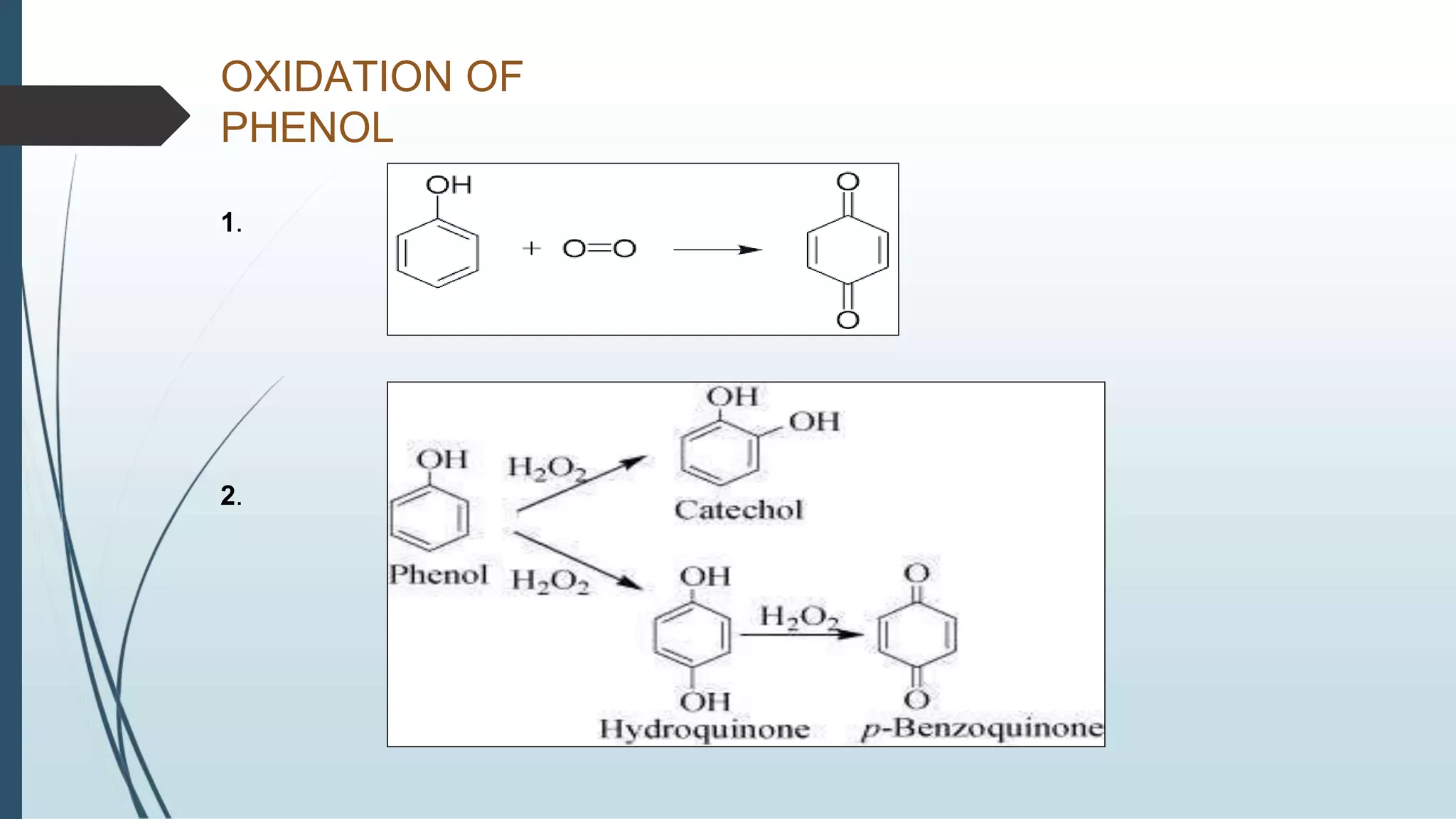
The document discusses the properties and reactions of phenols. Phenols contain a hydroxyl group directly attached to a benzene ring. They are more acidic than alcohols due to resonance stabilization of the phenoxide ion. Phenols undergo electrophilic aromatic substitution at the ortho- and para-positions. They are also oxidized by strong oxidizing agents. Common phenols include phenol, cresols, and resorcinol. Phenol is used as an antiseptic and in making resins, plastics and drugs. Cresols are used as herbicides, antioxidants and preservatives. Resorcinol is used as an antiseptic and disinfectant.