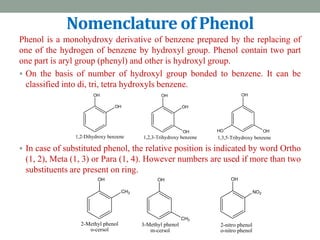
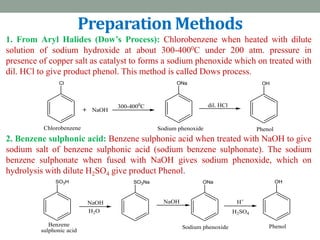
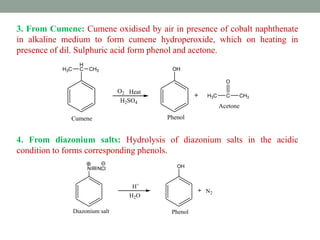
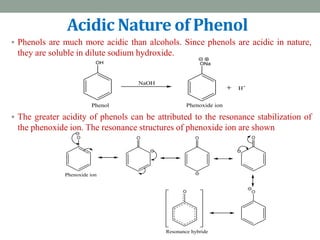
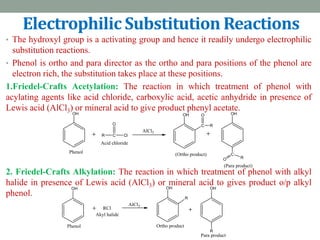
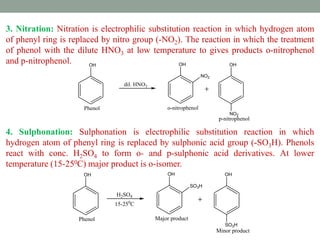
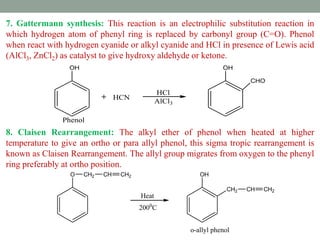
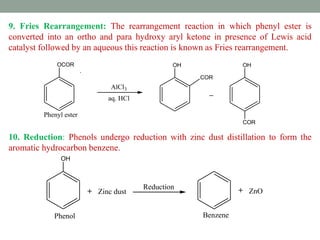
The document provides an extensive overview of phenols, including their classification, preparation methods, and physical properties. It explains the structure of phenol, the significance of the hydroxyl group, and details various chemical reactions involving phenols such as electrophilic substitution. The document also outlines specific reactions and their conditions, demonstrating the versatility and importance of phenols in organic chemistry.