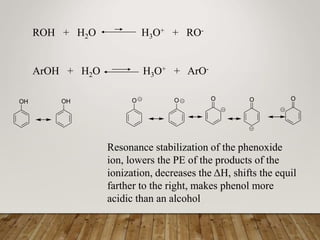
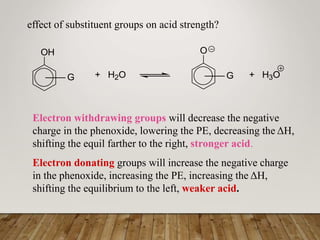
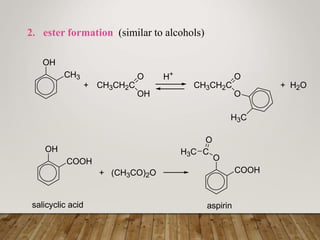
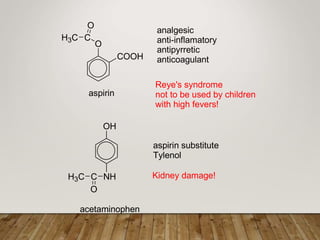
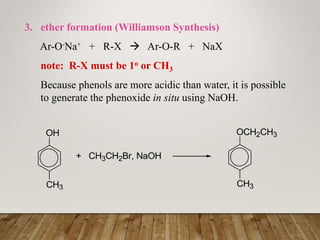
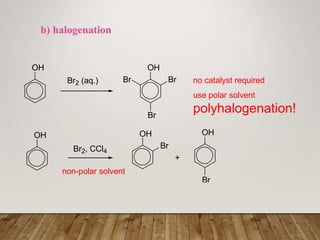
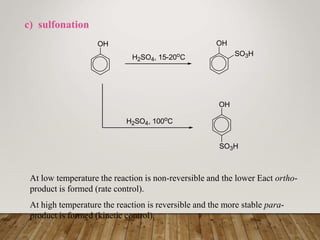
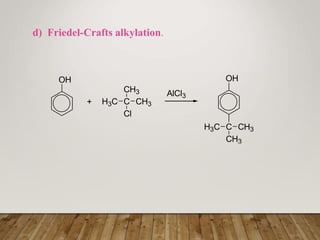
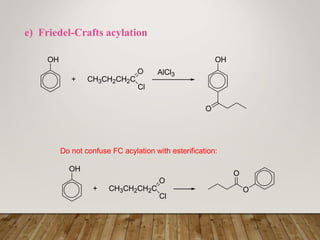
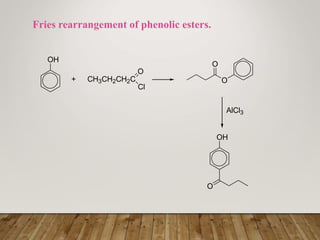
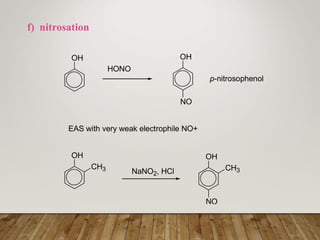
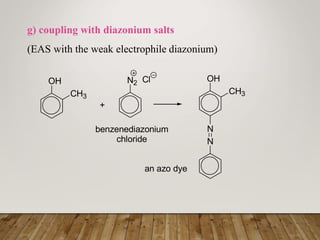
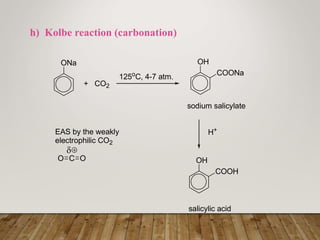
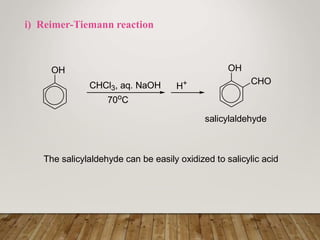
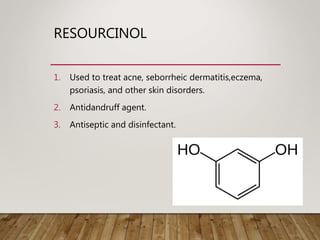
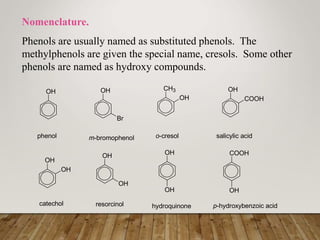
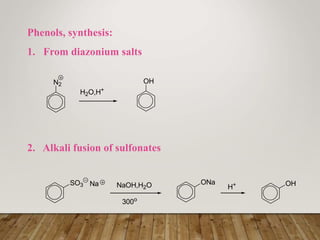
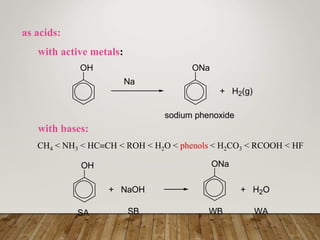
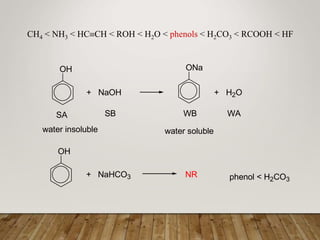
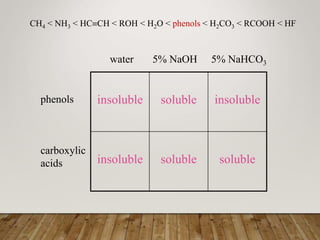
This document discusses phenols, which are aromatic compounds containing a hydroxyl (-OH) group. Phenols differ from alcohols in that the -OH group is attached to an aromatic carbon rather than an aliphatic carbon. Phenols are more acidic than alcohols due to resonance stabilization of the phenoxide ion. Phenols undergo electrophilic aromatic substitution reactions and reactions typical of alcohols like ester formation. Common phenols include phenol, cresols, resorcinol, and napthols, which are used to make plastics, drugs, dyes, disinfectants, and wood preservatives.




![We use the ionization of acids in water to measure acid
strength (Ka):
HBase + H2O H3O+ + Base-
Ka = [H3O+ ][ Base ] / [ HBase]
ROH Ka ~ 10-16 - 10-18
ArOH Ka ~ 10-10
Why are phenols more acidic than alcohols?](https://image.slidesharecdn.com/1288phenols-220920105734-886320be/85/1288_phenols-ppt-10-320.jpg)