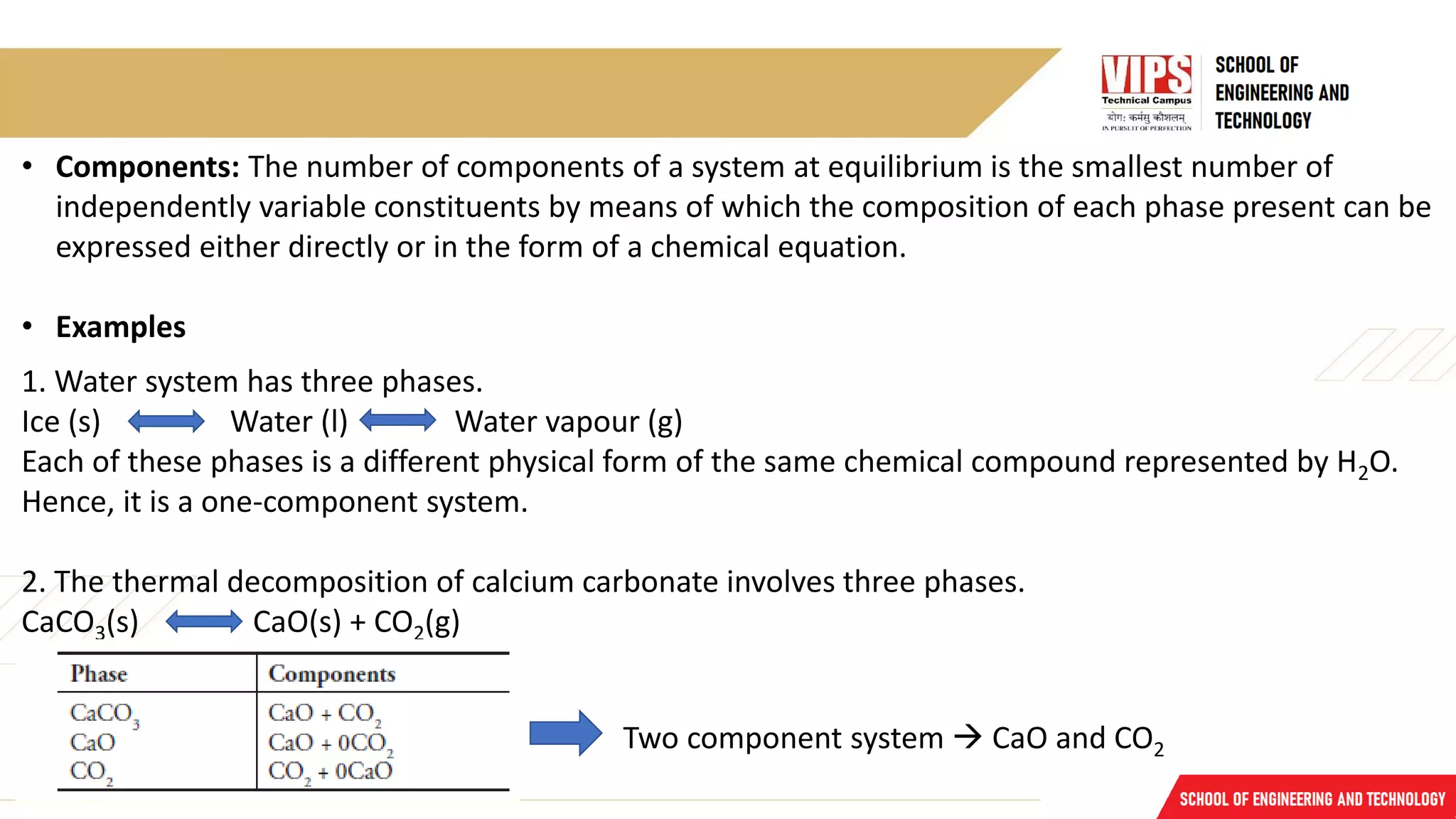
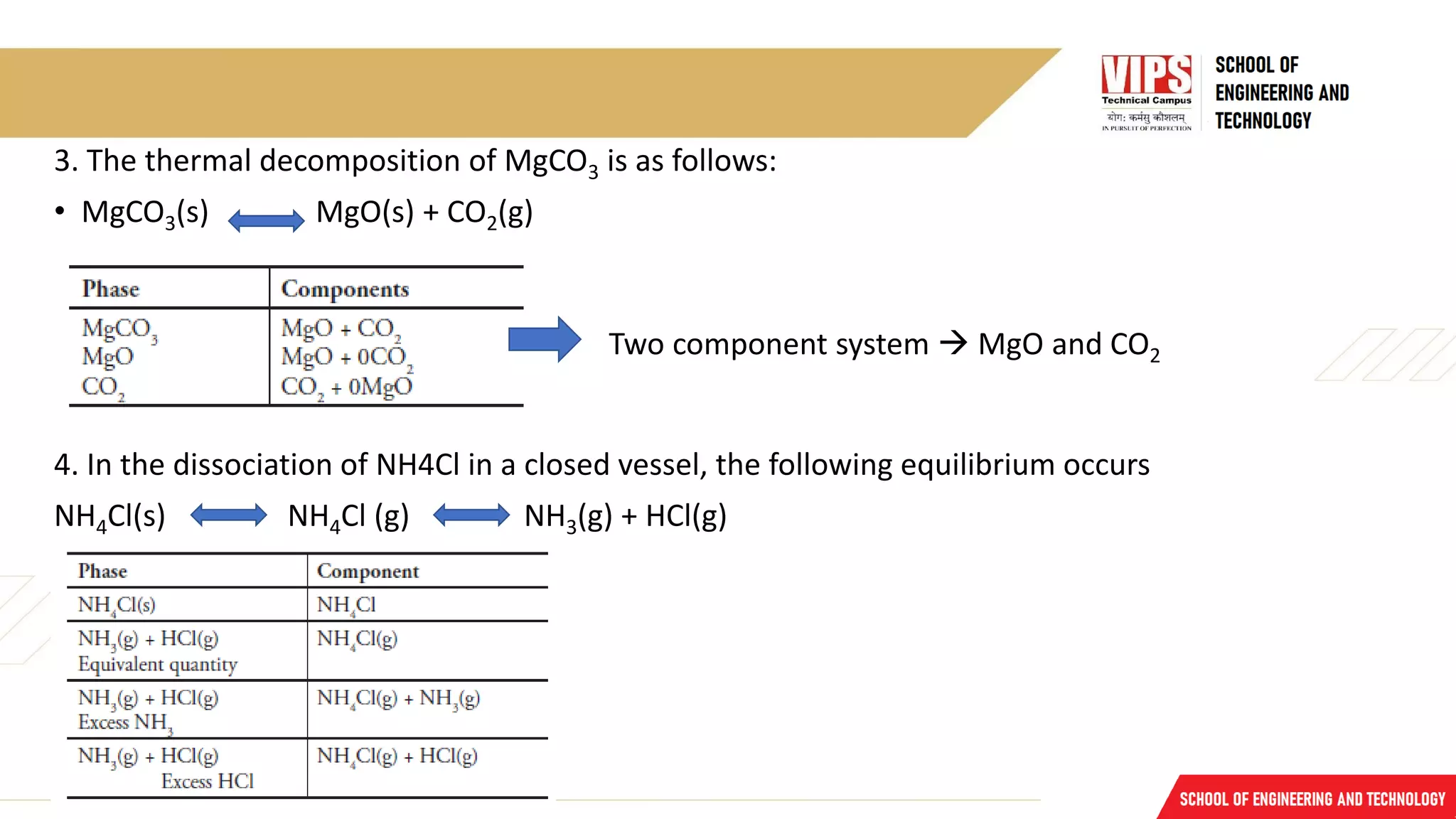
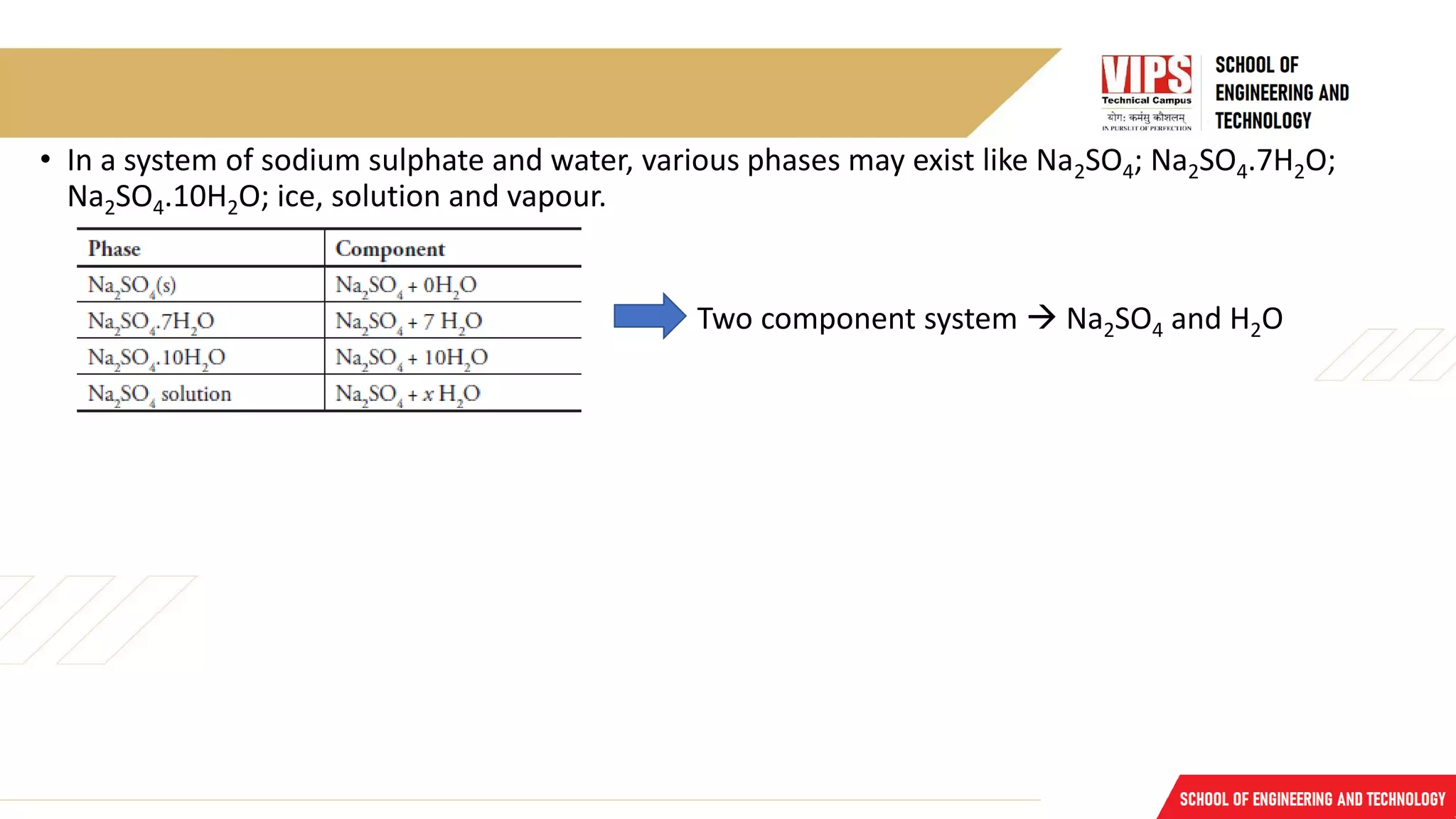
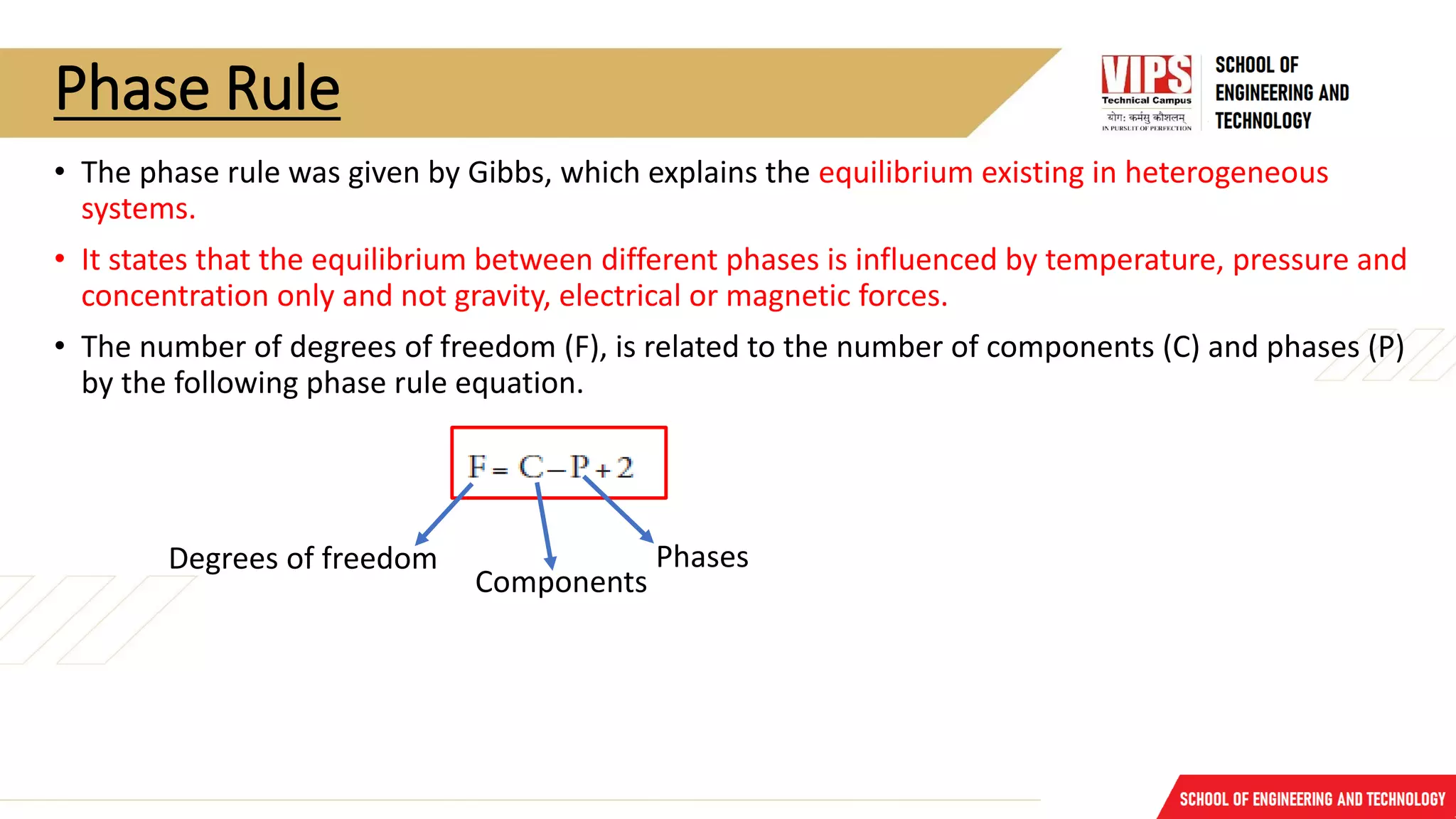
The document discusses phase rule and its applications. It defines a phase as a homogeneous and physically distinct portion of a system. The number of components in a system is the minimum number needed to express the composition of each phase. The degrees of freedom is the number of independent variables needed to define the system in equilibrium. The phase rule states that the number of degrees of freedom is equal to the number of components minus the number of phases plus two. Several examples of calculating the number of phases, components, and degrees of freedom in different chemical systems are provided.


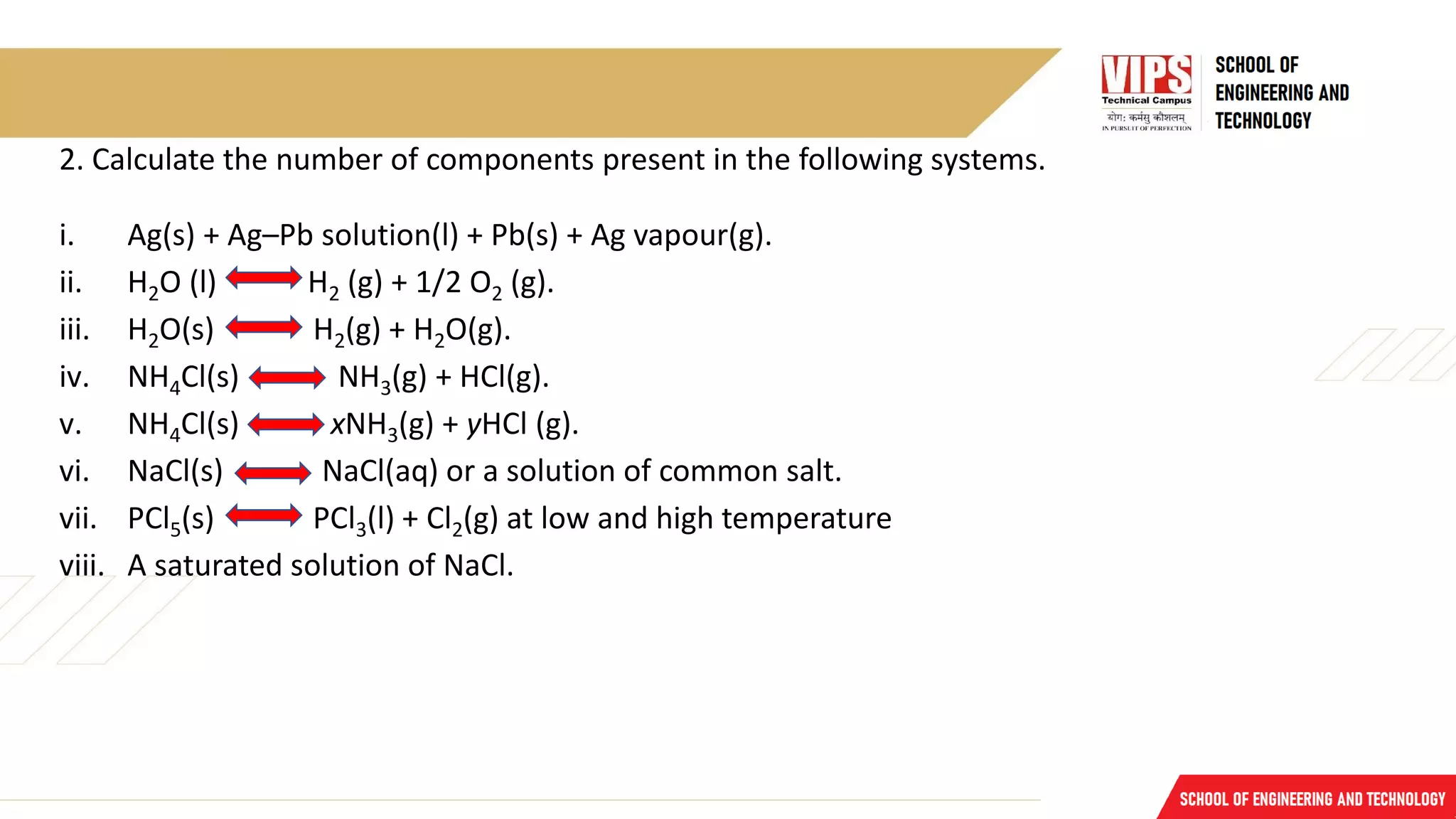
![Problems
1. Calculate the number of phases
i. CaCO3(s) CaO(s) + CO2(g).
ii. MgCO3(s) MgO(s) + CO2(g).
iii. Rhombic sulphur(s) Monoclinic sulphur(s).
iv. Ice (s) Water (l) Water vapour (g)
v. A system consisting of benzene and water.
vi. Closed beaker partially filled with benzene and water.
vii. Acetone and water.
viii. Fe(s) + H2O(g) FeO(s) + H2(g) equilibria.
ix. Solution of Mohr’s salt [FeSO4.(NH4)2SO4.6H2O].](https://image.slidesharecdn.com/lecture11-230117161551-f8afd52c/75/Lecture-11-pdf-7-2048.jpg)
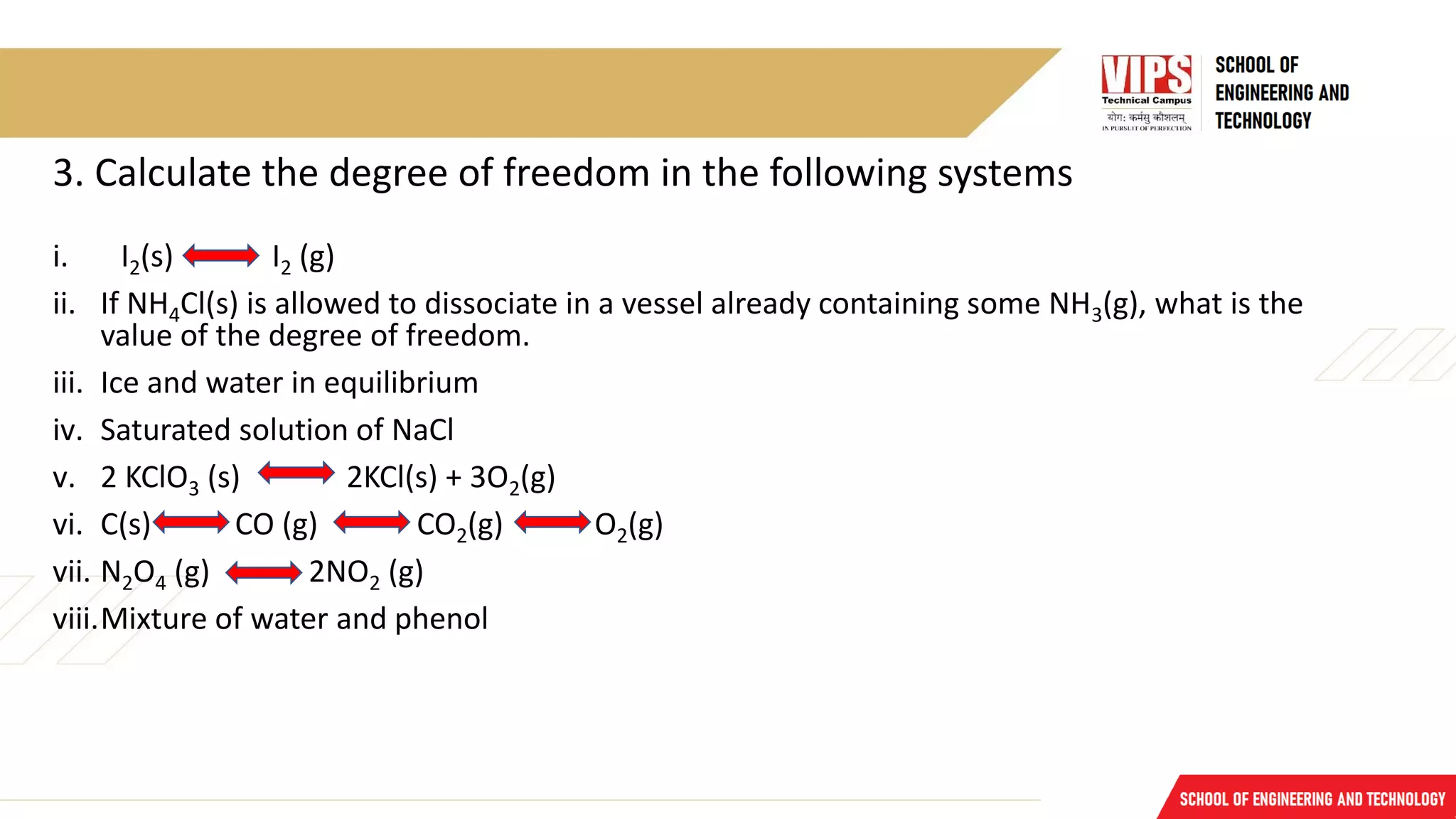
![Solution for Q3
i. C =1; P =2
F = C – P + 2; F = 1 – 2 + 2 = 1
ii. F = C – P + 2
C = 2 (NH4Cl + NH3); P = 2 [NH4Cl(s) + {NH4Cl(g) & NH3(g) } ]
F = 2 – 2 + 2 = 2
iii. P = 2; C = 1; F = C – P + 2; F = 1 – 2 + 2 = 1
iv. C = 2, P = 3; F = C – P + 2; F = 2 – 3 + 2 = 1
v. P = 3, C = 2; F = C – P + 2; F = 2 – 3 + 2 = 1
vi. Phases = 2 (solid phase due to carbon + gaseous phase due to CO2 + CO + O2
C = 2 (carbon + oxygen)
F = C – P + 2; 2 – 2 + 2 = 2
vii. P = 1, C = 1; F = C – P + 2 = 1 – 1 + 2 = 2
viii. P = 2, C = 2; F= C – P + 2 = 2 – 2 + 2 = 2](https://image.slidesharecdn.com/lecture11-230117161551-f8afd52c/75/Lecture-11-pdf-11-2048.jpg)
