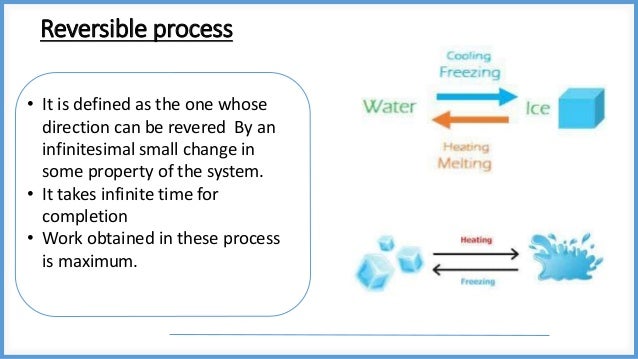
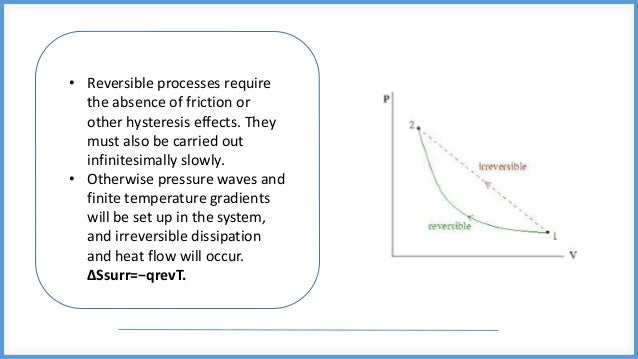
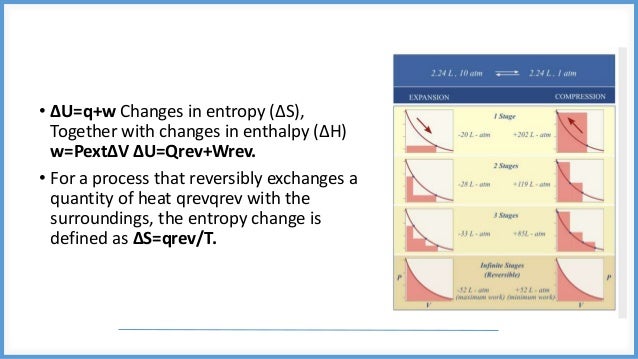
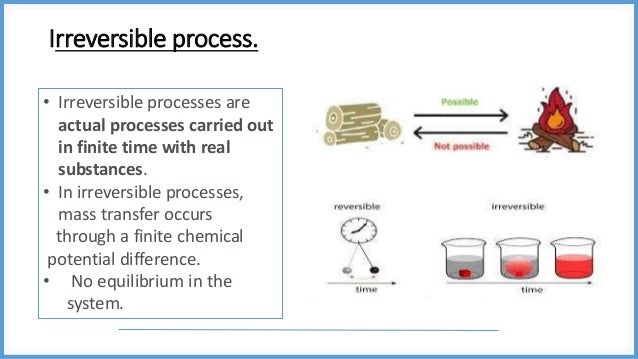
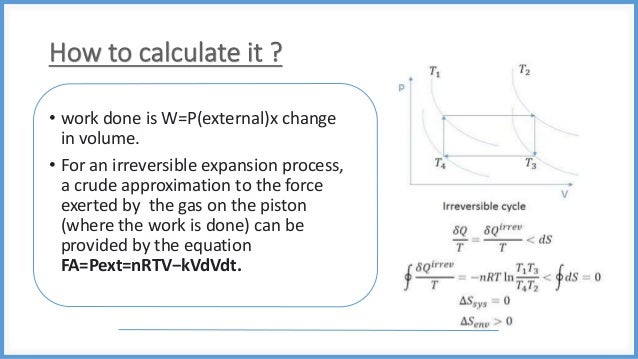
This document discusses reversible and irreversible processes in thermodynamics. It defines a reversible process as one whose direction can be reversed by an infinitesimal change, requiring infinite time and resulting in maximum work. Reversible processes must be carried out infinitely slowly to avoid irreversible dissipation and heat flow. For a reversible process exchanging heat with the surroundings, the entropy change is defined as the heat transfer divided by temperature. Irreversible processes are actual processes carried out in finite time that involve nonequilibrium states and crude force approximations. A reversible process allows the system and environment to return to their original states, while an irreversible process does not.