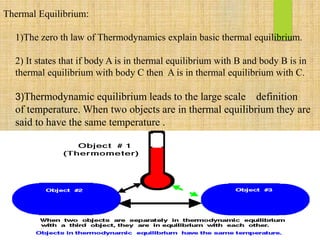
This document discusses thermodynamic equilibrium, states, and phases. It defines thermodynamic equilibrium as a state where a system achieves thermal, chemical, and mechanical balance, with nothing changing at the macroscale. Thermal equilibrium occurs when two objects have the same temperature, and chemical equilibrium is a state where reactants and products are present at constant concentrations. A system's state is defined by variables like temperature, pressure, and volume, and a phase is a physically distinct, chemically homogeneous portion of a system. The phase rule relates the number of degrees of freedom in a system to the number of components and phases present.