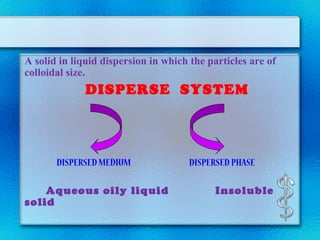
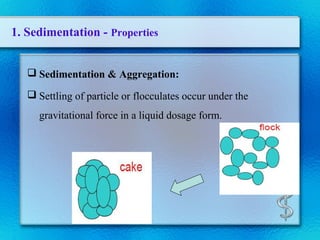
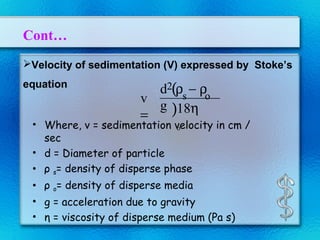
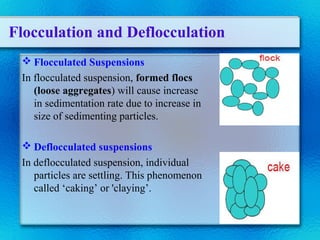
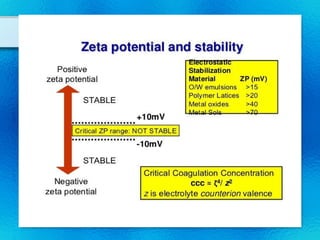
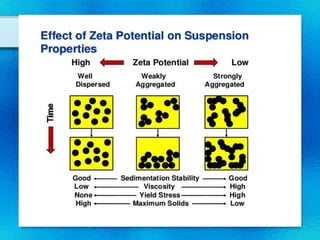
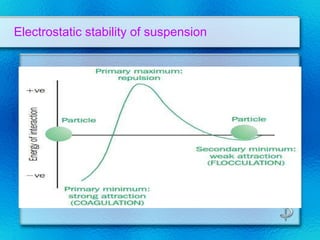
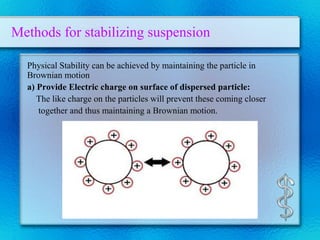
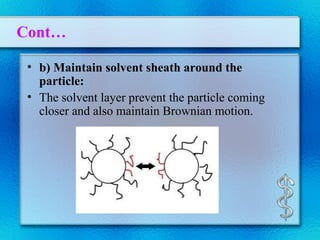
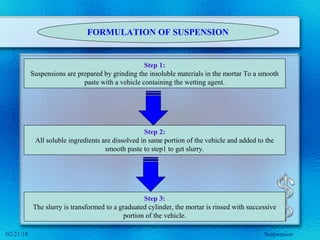
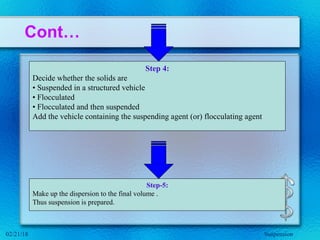
This document discusses suspensions, which are heterogeneous systems consisting of solid particles dispersed throughout a liquid. It defines suspensions and describes their classification based on particle size. Key points include that suspensions have solid particles too large to be considered a true solution, stability is important to prevent settling or caking, and suspending agents are often added to help disperse particles and maintain stability. The document also covers formulation considerations like choice of vehicle and processing steps.