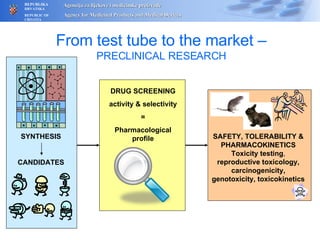
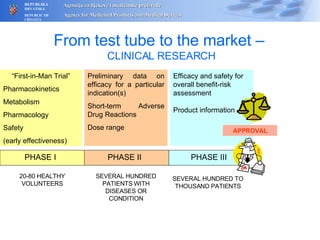
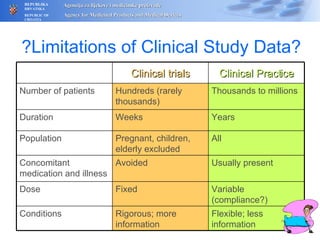
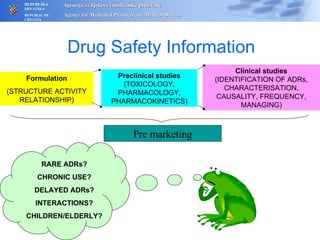
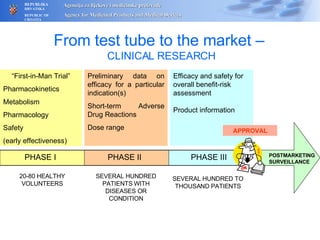
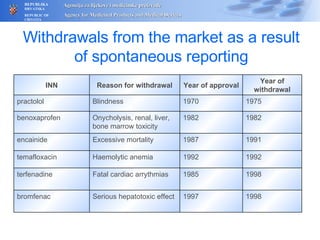
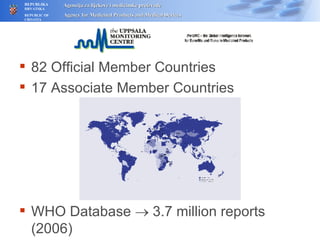
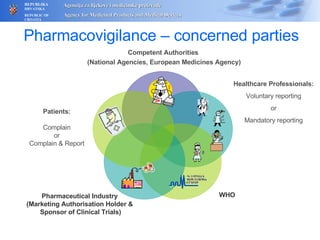
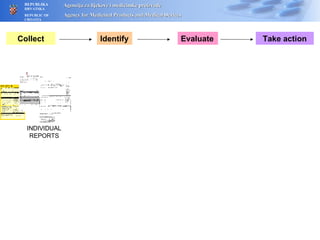
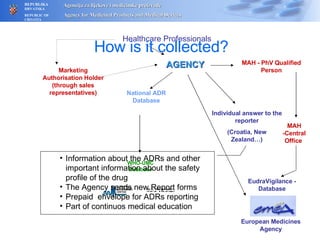
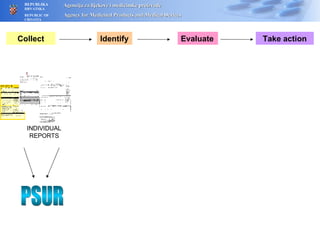
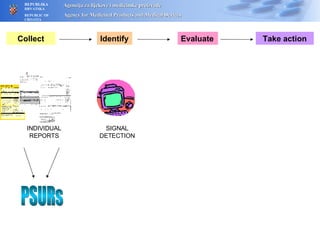
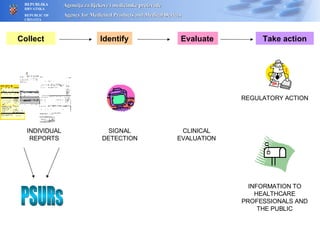
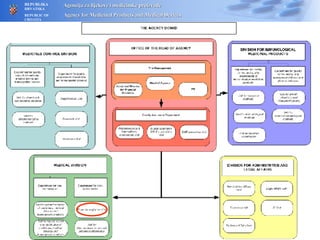
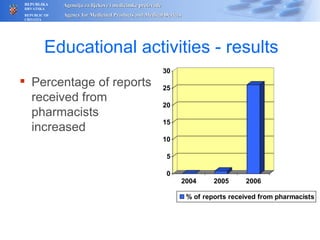
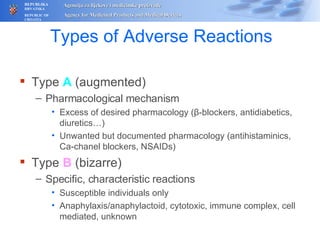
The document outlines a pharmacovigilance workshop, including definitions of pharmacovigilance, the need for monitoring drug safety post-approval, and the pharmacovigilance system process of collecting, evaluating and taking action on adverse drug reactions. The workshop introduction discusses educational activities in Croatia to increase healthcare professional reporting of adverse reactions.