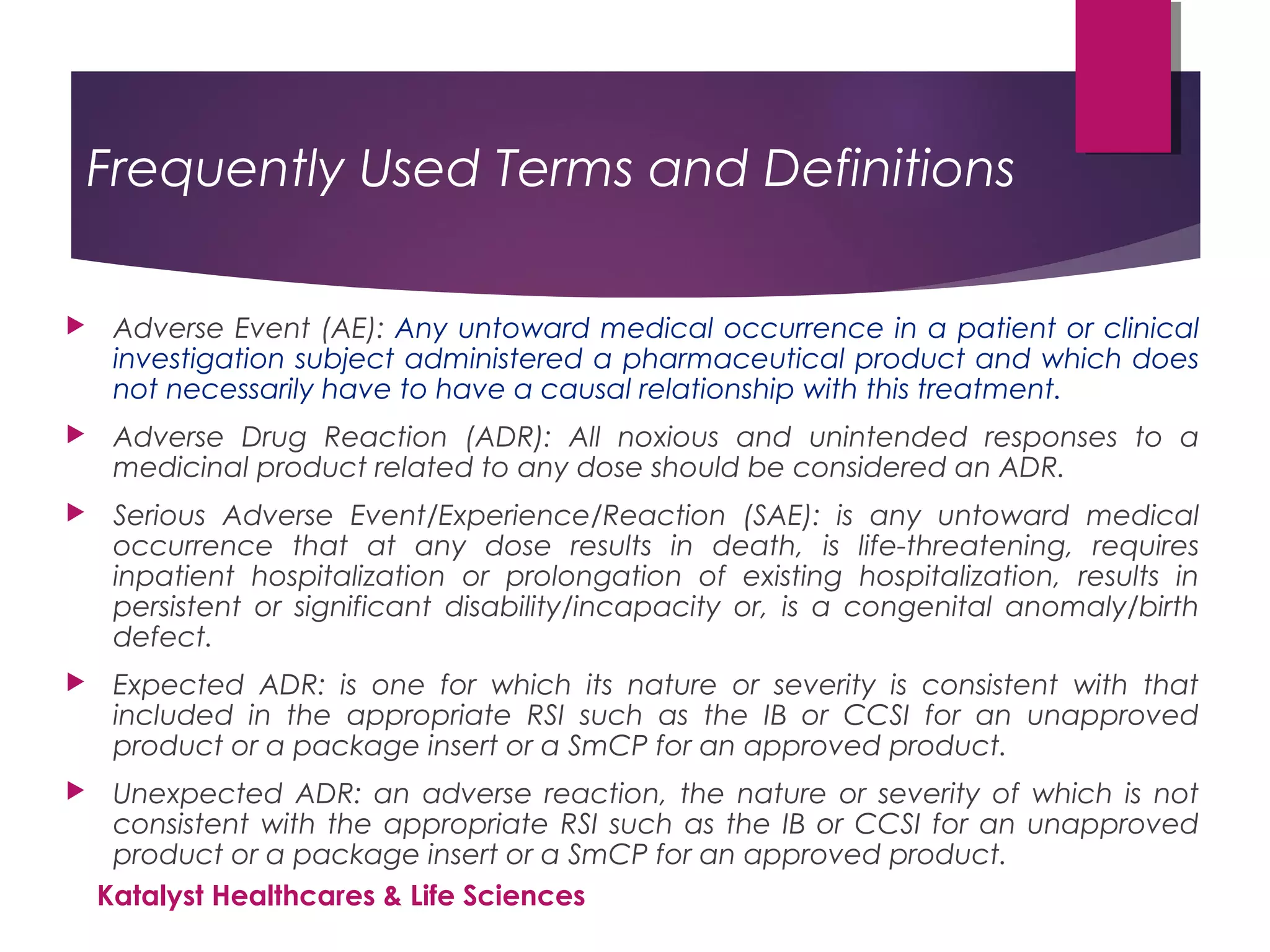
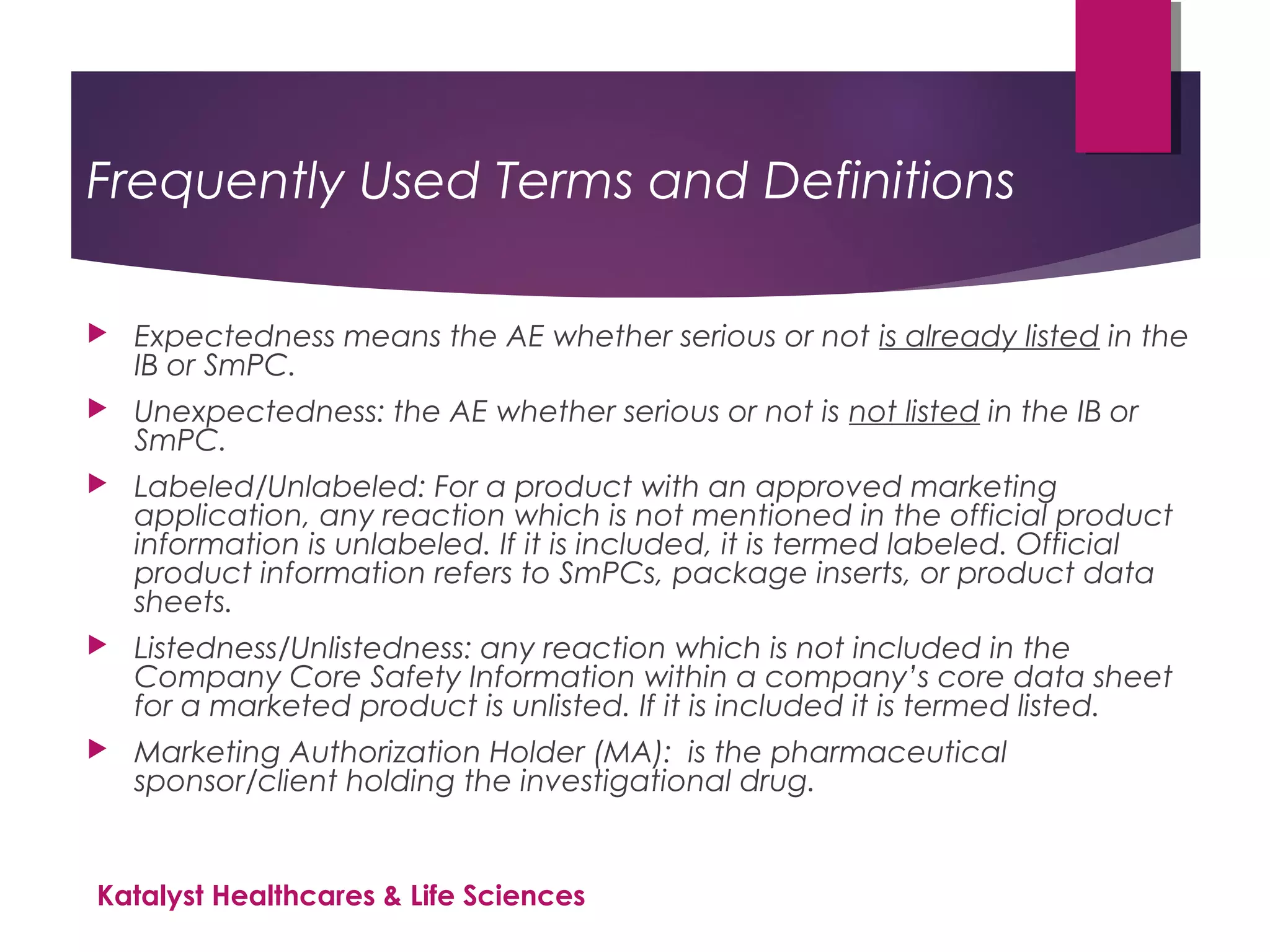
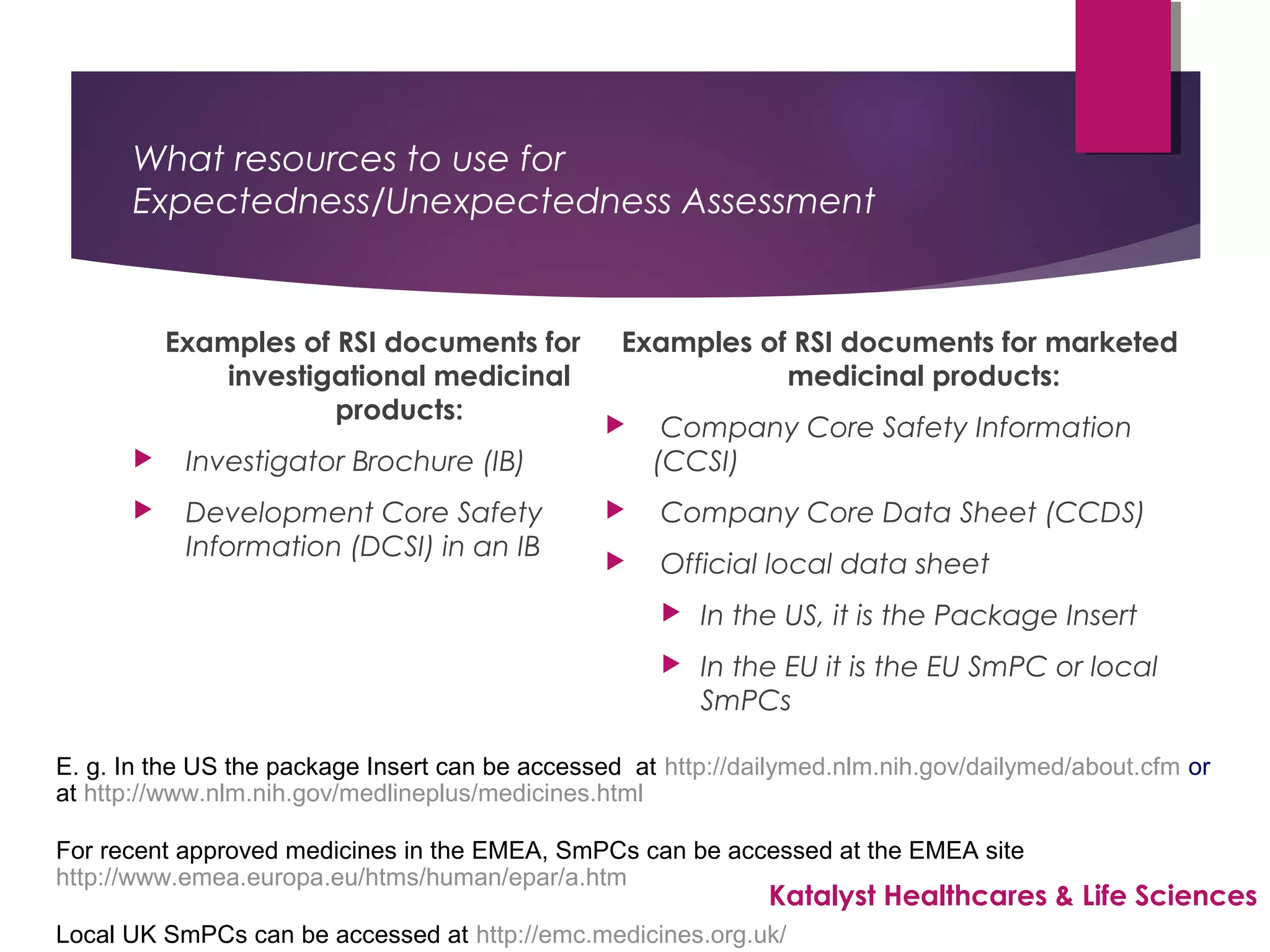
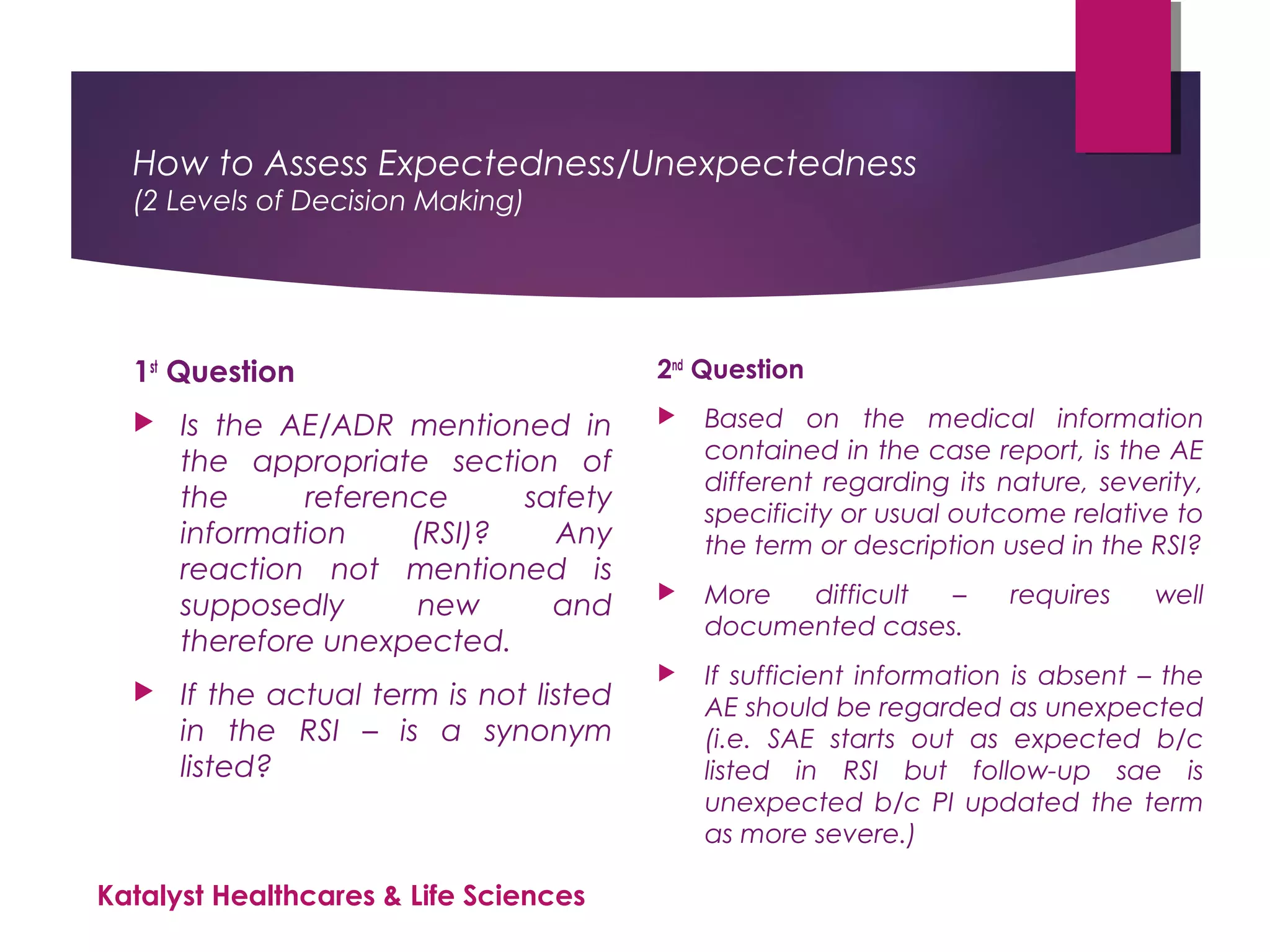
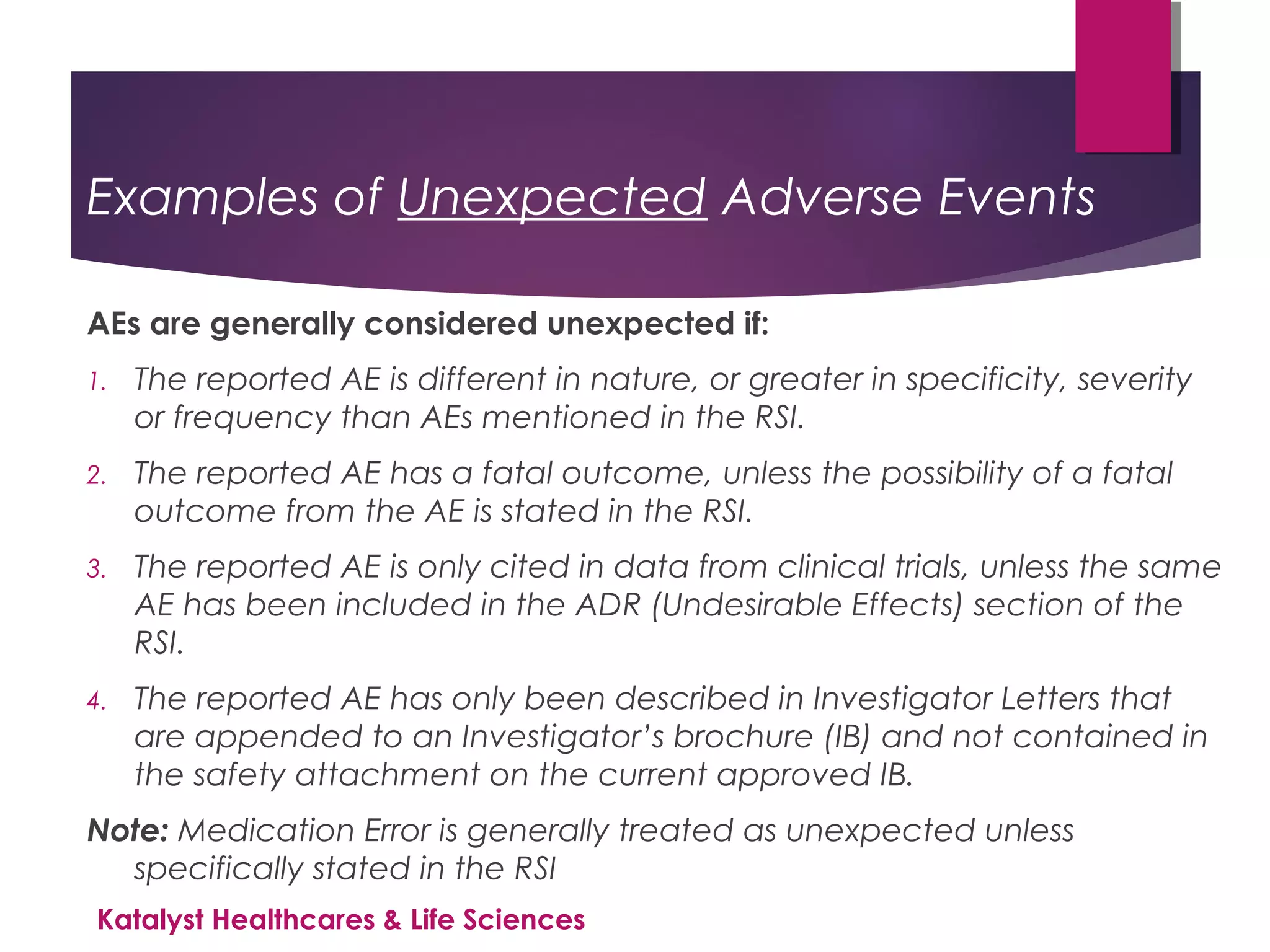
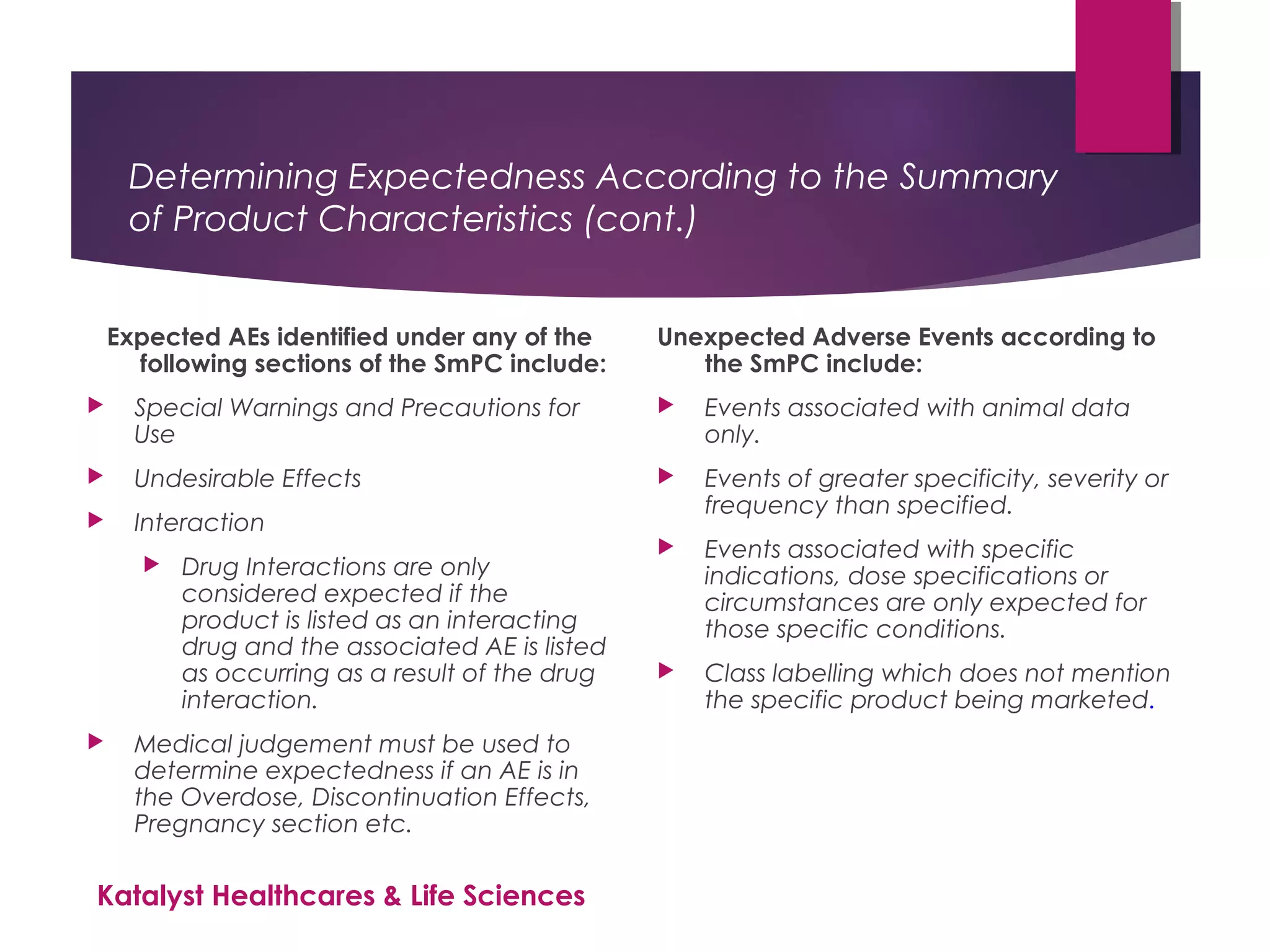
The document outlines the training objectives and essential terminologies related to expectedness and unexpectedness assessments for adverse events in drug safety. It provides definitions, examples, and guidelines for determining the classification of adverse events based on reference safety information. The importance of these assessments is emphasized in relation to regulatory reporting requirements and pharmacovigilance practices.











![Helpful CIOMS Rules[1]
Further anatomical specification of a labelled AE does NOT make it
unlabelled:
E.g., fibrosis of upper left lobe is equivalent to lung fibrosis
However: if arteritis is expected, temporal arteritis should be considered
unexpected due to the associated additional risks and poorer prognosis
Extra histological specification does NOT make, per se, a labelled AE
unlabeled:
E.g., liver biopsy shows hepatic necrosis [labelled] with the presence of
eosinophiles [not mentioned in labelling]
However: e.g., cerebral thrombo-embolism or cerebral vasculitis would
be unexpected [by virtue of greater specifity] if the labelling only listed
cerebral vascular accidents.
Interstitial nephritis should be considered unexpected when only acute
renal failure is expected.
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-18-2048.jpg)
![Helpful CIOMS Rules [2] cont.
If a labelled AE is not normally accompanied by an additional sign
or symptom, AE should NOT be considered labelled:
E.g., if the labelling mentions gastrointestinal irritation, then
‘gastrointestinal irritation associated with melena’ would be
unlabelled.
Mention of any additional symptom or sign usually associated with
an already labelled AE does NOT merit upgrading the event to
unlabelled:
E.g., labelling mentions thrombocytopenia, then a report on
‘thrombocytopenia associated with petechia’ would be labelled;
E.g., labelling mentions pseudomembraneous colitis, then a
report on ‘pseudomembraneous colitis with dehydration would
be labelled;
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-19-2048.jpg)
![Helpful CIOMS Rules [3] cont.
CIOMS rule: In general, the medical view is that if a labelled AE is
often life-threatening or often results in death, a fatal outcome in a
particular case does NOT make the AE unlabeled, even if death is
NOT mentioned in the labelling as possible outcome:
E.g., myocardial infarction is mentioned, but fatal myocardial
infarction is not.
However, as a policy decision, company adopted the overall
conservative approach for world-wide reporting, that all fatal
outcomes are assessed as unexpected, unless explicitly
mentioned in the RSI.
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-20-2048.jpg)
![Helpful CIOMS Rules [3] cont.
If a reported AE is significantly more severe than the labelled AE, it
should be considered unlabeled:
E.g., circulatory collapse would be unlabelled when hypotension
is labelled
E.g., death from hepatic necrosis would be unlabelled when
hepatic failure is labelled
Rash does cover morbiliform rash, but not Steven Johnson
Syndrome
Fulminant hepatitis should not be considered expected if only
‘liver injury’ is mentioned
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-21-2048.jpg)
![Helpful CIOMS Rules [4] cont.
If an AE is not medically more important than a labelled AE, the case
need not be considered unlabelled:
E.g., vertigo, when dizziness is labelled;
E.g., raised liver function test when hepatitis is labelled
Death from a condition diagnosed prior to treatment is NOT a
reportable event – in fact, it is not an event at all !
E.g., death in pre-existing bronchogenic carcinoma
Exception: exacerbation of the pre-existing condition following
treatment leading to death
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-22-2048.jpg)
![Helpful CIOMS Rules [4] cont.
AE with causality disclaimer – are considered expected (including
those listed in a frequency table).
E.g., "The following adverse events have been reported in
association with the drug, but a causal relationship has not been
established”
However: if the AE disclaimer states that the events have been
reported but not been considered drug-related (negative
causality statement), then the AE should be assessed as
unexpected
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-23-2048.jpg)
![Helpful CIOMS Rules [5] cont.
An unlabelled diagnosis which relates to a group of symptoms or
signs which are labelled, the new case is not in itself labelled:
E.g., anaphylaxis is unlabelled, even if hypotension, wheezing
and urticaria are all labelled (DIAGNOSIS must be stated in the
label to make it expected)
If a DIAGNOSIS is labelled, then the signs and symptoms which
comprise the diagnosis are also considered to be labelled.
E.g., anaphylaxis is labelled, then hypotension, wheezing and
urticaria together would be considered to be labelled
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-24-2048.jpg)
![Helpful CIOMS Rules [5] cont.
HOWEVER: Even though a diagnosis or syndrome is expected, if
the usually accompanying signs and symptoms are reported in
the absence of a clear diagnosis (i.e., as one or more isolated
signs and symptoms), those terms should not be considered as
expected unless already in the RSI. It is impossible to ascertain
that their appearance alone or together necessarily reflects a
mechanism similar to that of a labelled diagnosis (e.g., isolated
nausea, or asthenia, or gastralgia, when liver injury is labelled;
isolated pallor, or hypotension or pruritus when anaphylactic
reaction is labelled).
If the label lists an AE which is specified to be transient, but it persists
in the new case, the case is unlabeled and should be reported:
E.g., prolonged elevated liver function tests, when labelling states
transient elevated liver function tests
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-25-2048.jpg)
![Helpful CIOMS Rules [6] cont.
If an AE/ADR has been reported only in association with an
overdose, then that same AE/ADR at usual doses should be
considered unexpected.
If an AE/ADR occurs in a different indication, it is assessed
unlabelled unless it is described in the adverse events section
applicable for a specific indication or patient population.
If an AE/ADR follows a different route/formulation of drug
administration, the event labelled for one presentation cannot be
considered labelled for another presentation.
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-26-2048.jpg)
![Helpful CIOMS Rules [6] cont.
Drug exposure during pregnancy: Abortion, stillbirth, congenital
abnormalities and maternal/new-born hazards are considered
unlabelled unless explicitly specified in the RSI.
Pregnancy/drug exposure in utero or normal babies are considered
labelled.
Note: Pregnancy, drug exposure in utero, and delivery of a healthy
newborn are no adverse events per se!
Drug abuse, drug dependence, maladministration: unlabelled
unless explicitly specified in the relevant sections of the RSI.
Lack of efficacy and resulting signs or symptoms are considered
labelled events. However, if treatment directly exacerbates the
treated condition then exacerbation is considered unlabelled
unless specifically mentioned in the RSI.
If an AE/ADR is due to a specific drug-drug interaction, it is
considered labelled but only in the context of the drug-drug
interaction.
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-27-2048.jpg)
![Helpful CIOMS Rules [7] cont.
Death as an Outcome:
Unless the RSI specifies a fatal outcome, then the case should be
considered as unexpected as long as there was an association between
the adverse reaction and the fatality
A fatal outcome to a suspected ADR should not be mentioned in the RSI
unless it has been reported to occur and is thought to be causally
related to the ADR.
In the absence of special circumstances, once the fatal outcome is itself
expected (labelled/listed), reports involving fatal outcomes should be
handled as for any other serious suspected ADR in accord with
appropriate regulatory requirements.
Katalyst Healthcares & Life Sciences](https://image.slidesharecdn.com/expectednesskatalysthls-170224042020/75/Expectedness-Unexpectedness-Assessment_Katalyst-HLS-28-2048.jpg)

