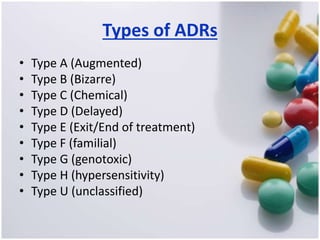
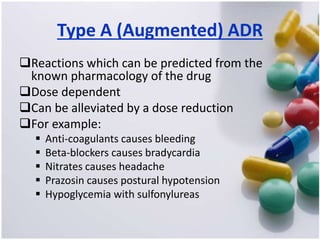
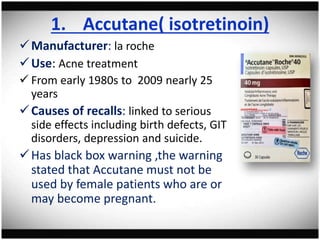
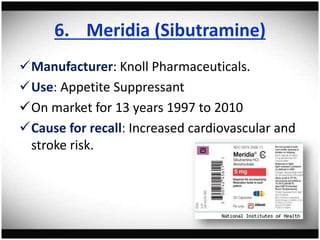
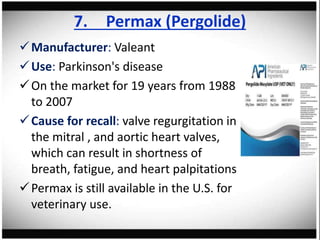
The document discusses pharmacovigilance, its significance in monitoring drug safety, and the establishment of the Egyptian Pharmacovigilance Center. It outlines the types of adverse drug reactions (ADRs) and the reporting processes in Egypt, emphasizing the roles of healthcare professionals, particularly pharmacists, in ensuring patient safety. The document also highlights challenges in pharmacovigilance like lack of awareness, reporting issues, and the impact of drug regulations such as FDA recalls.