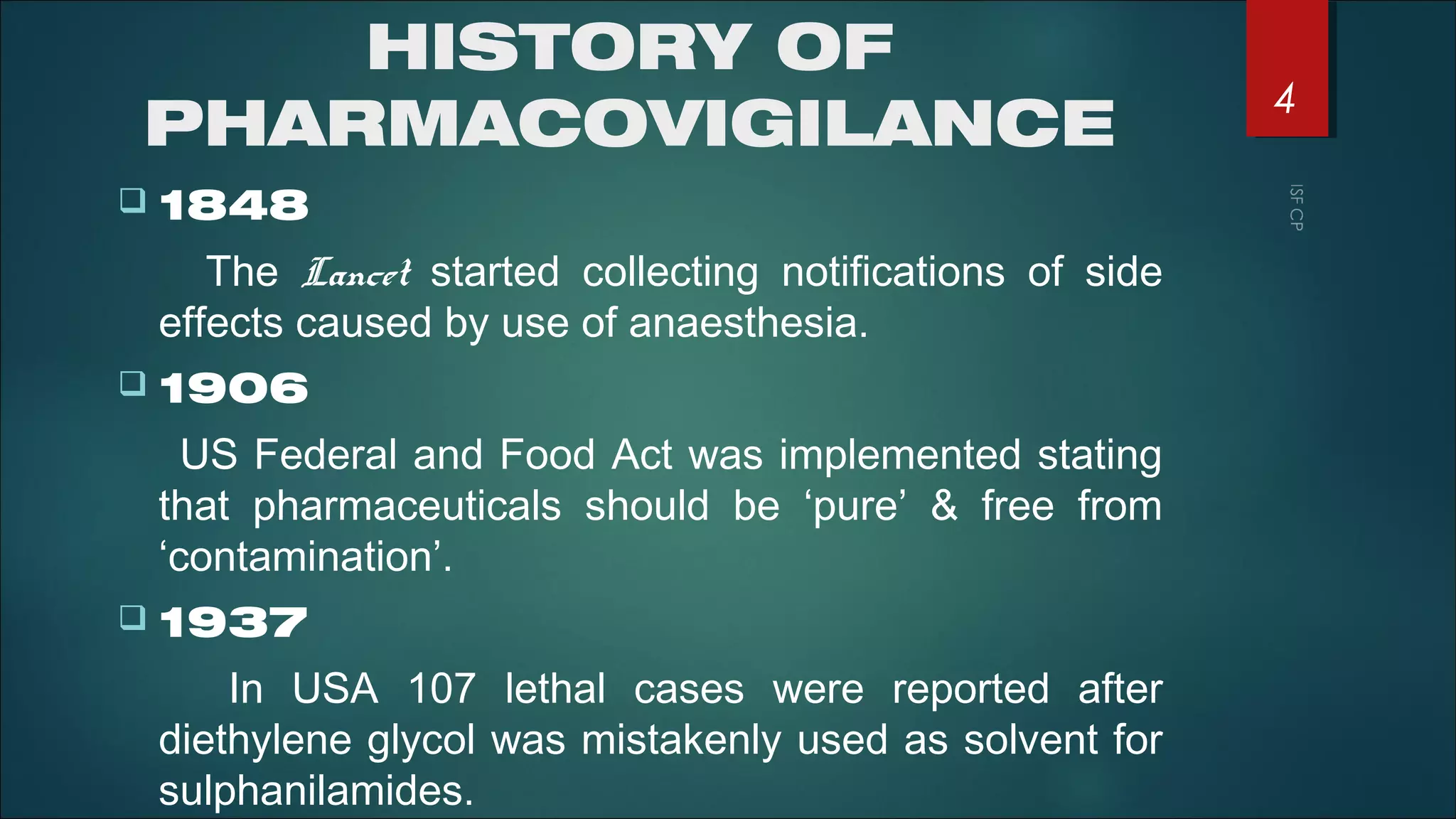
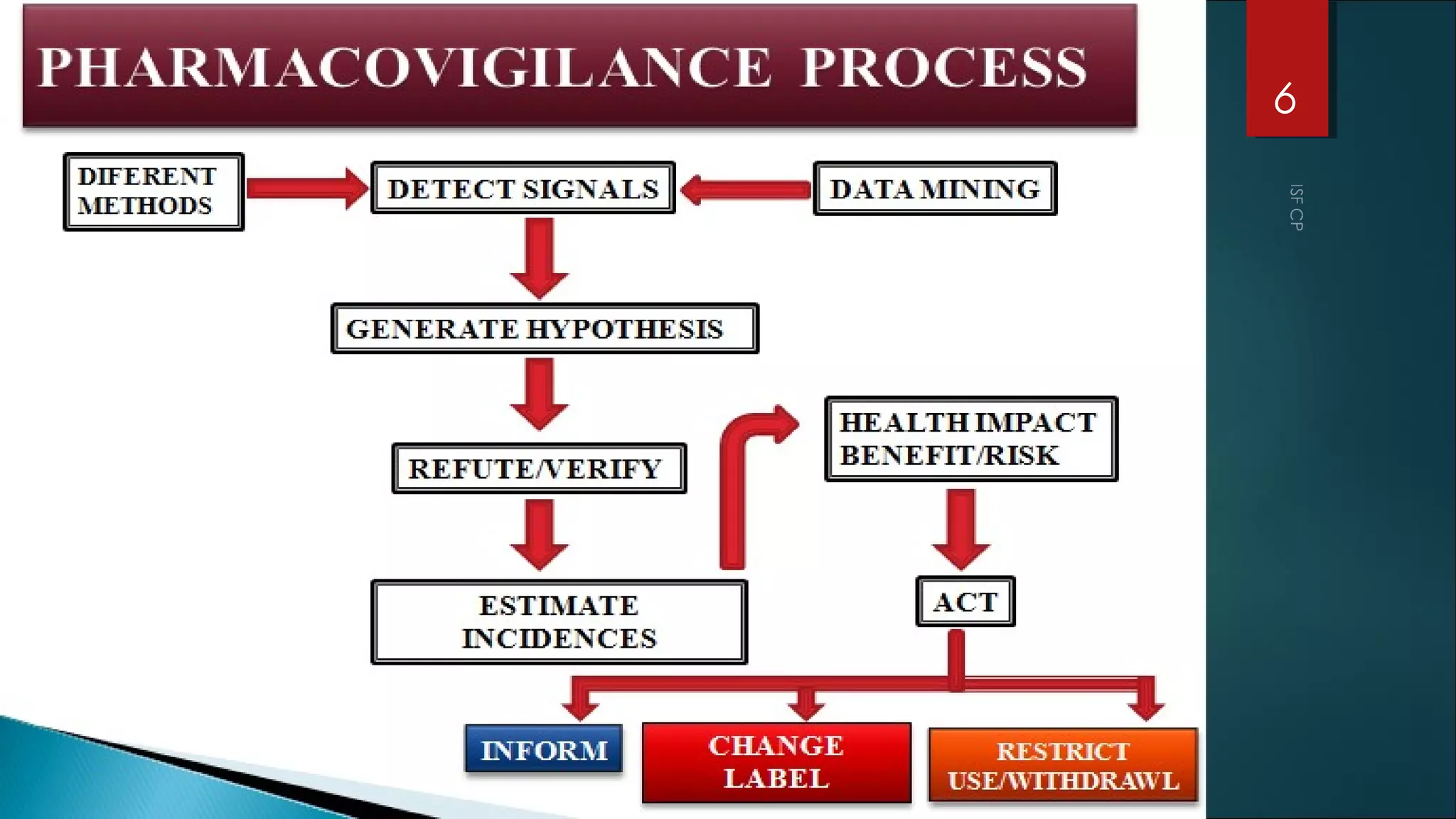
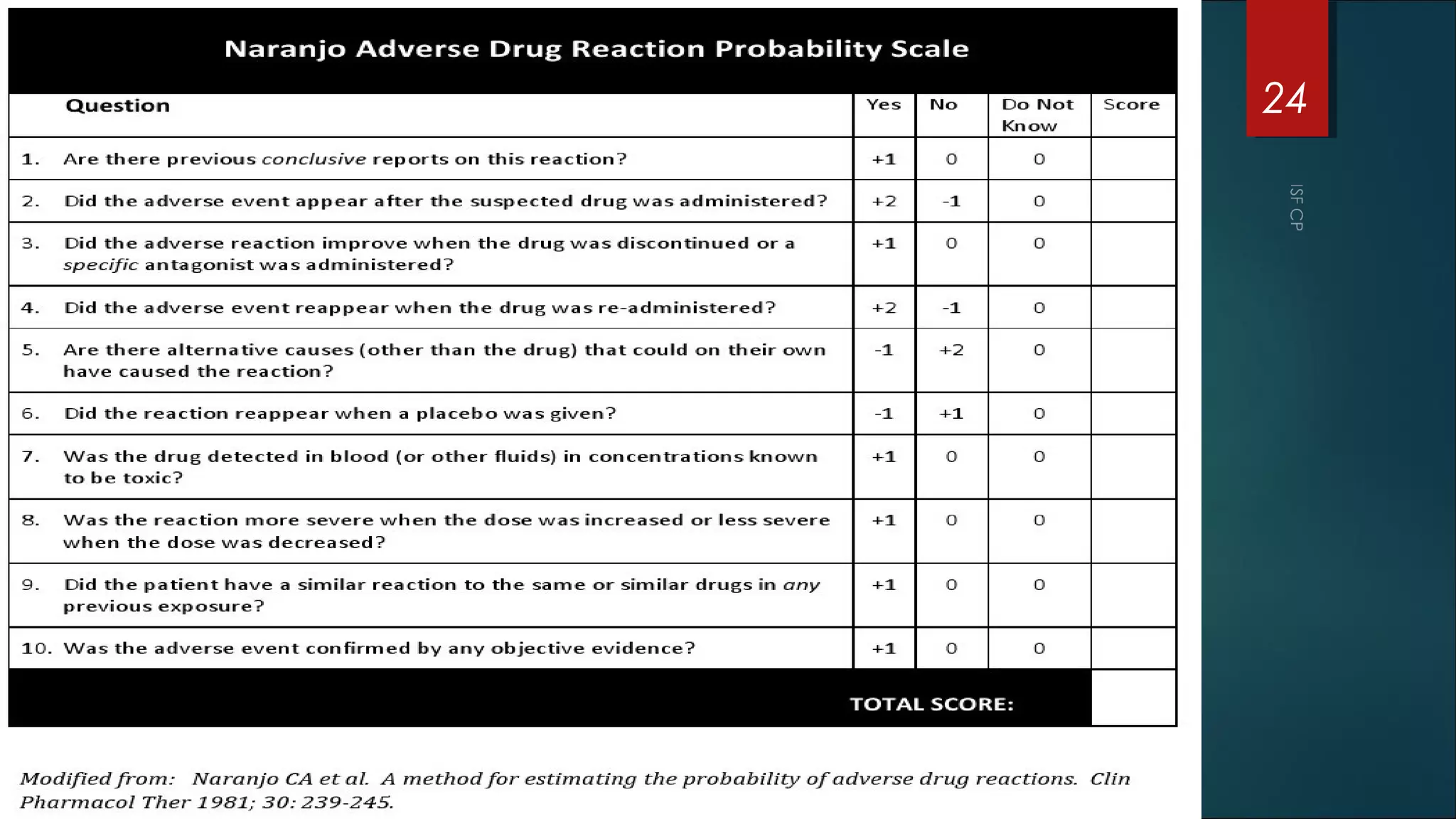
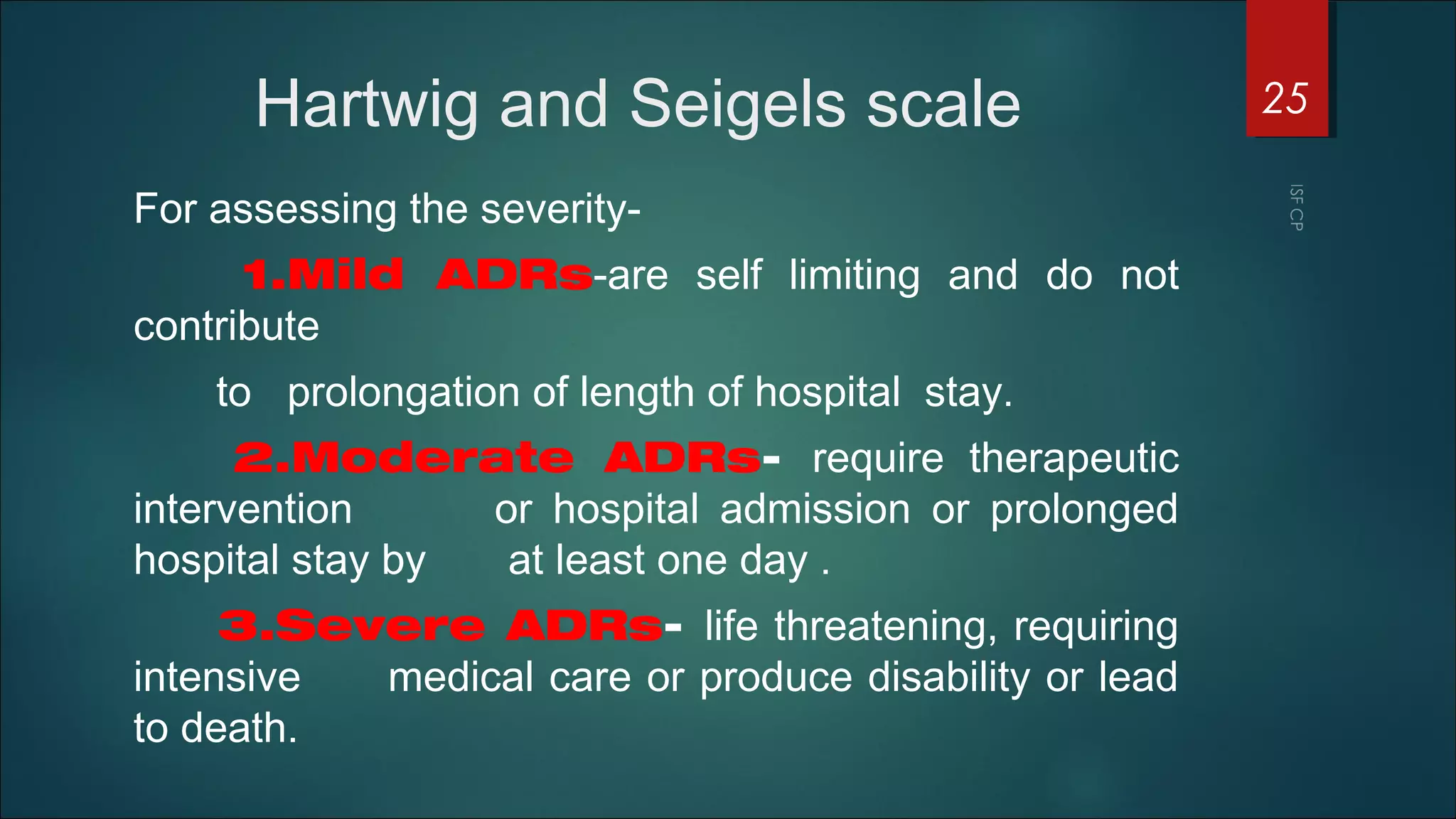
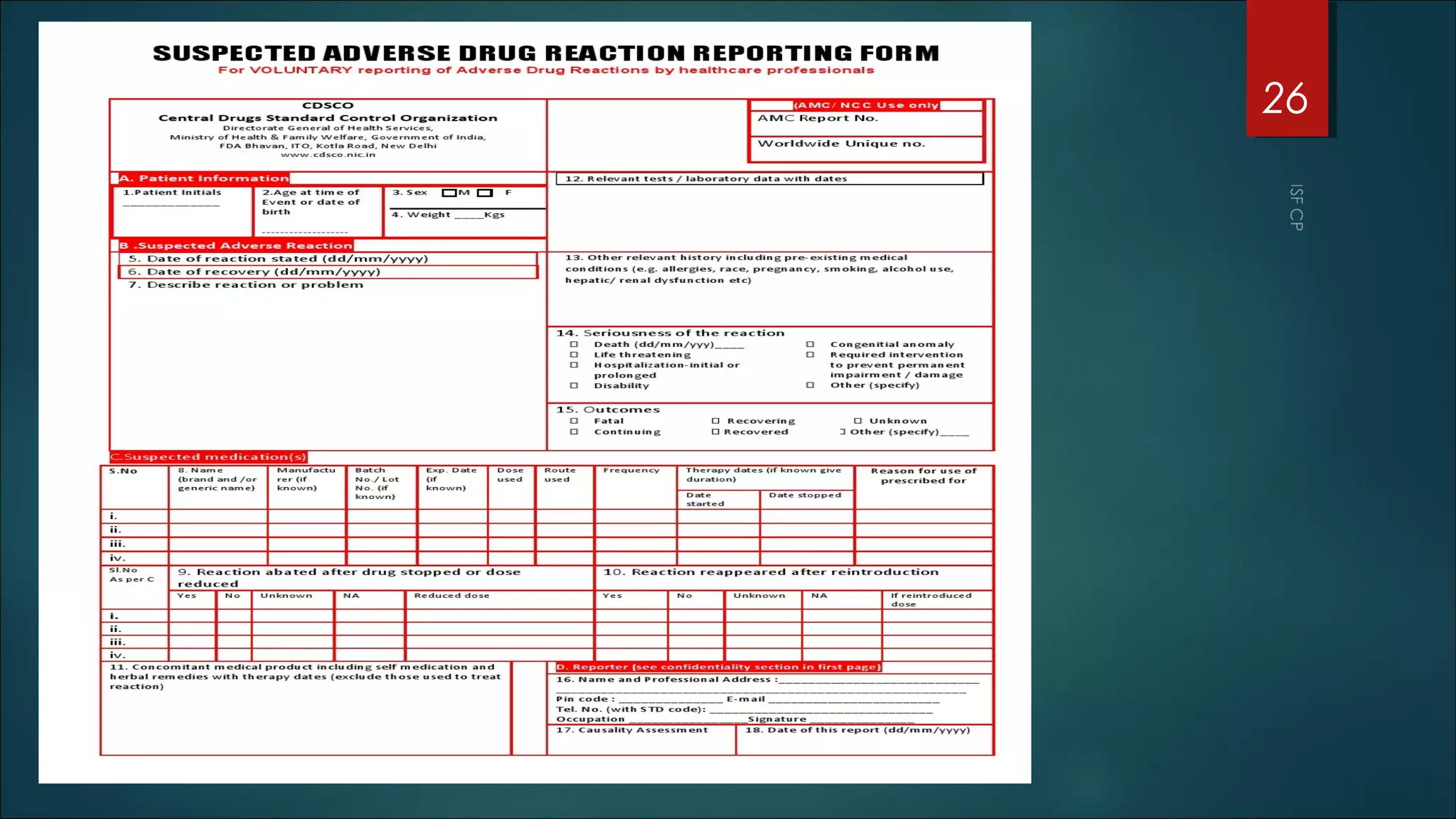
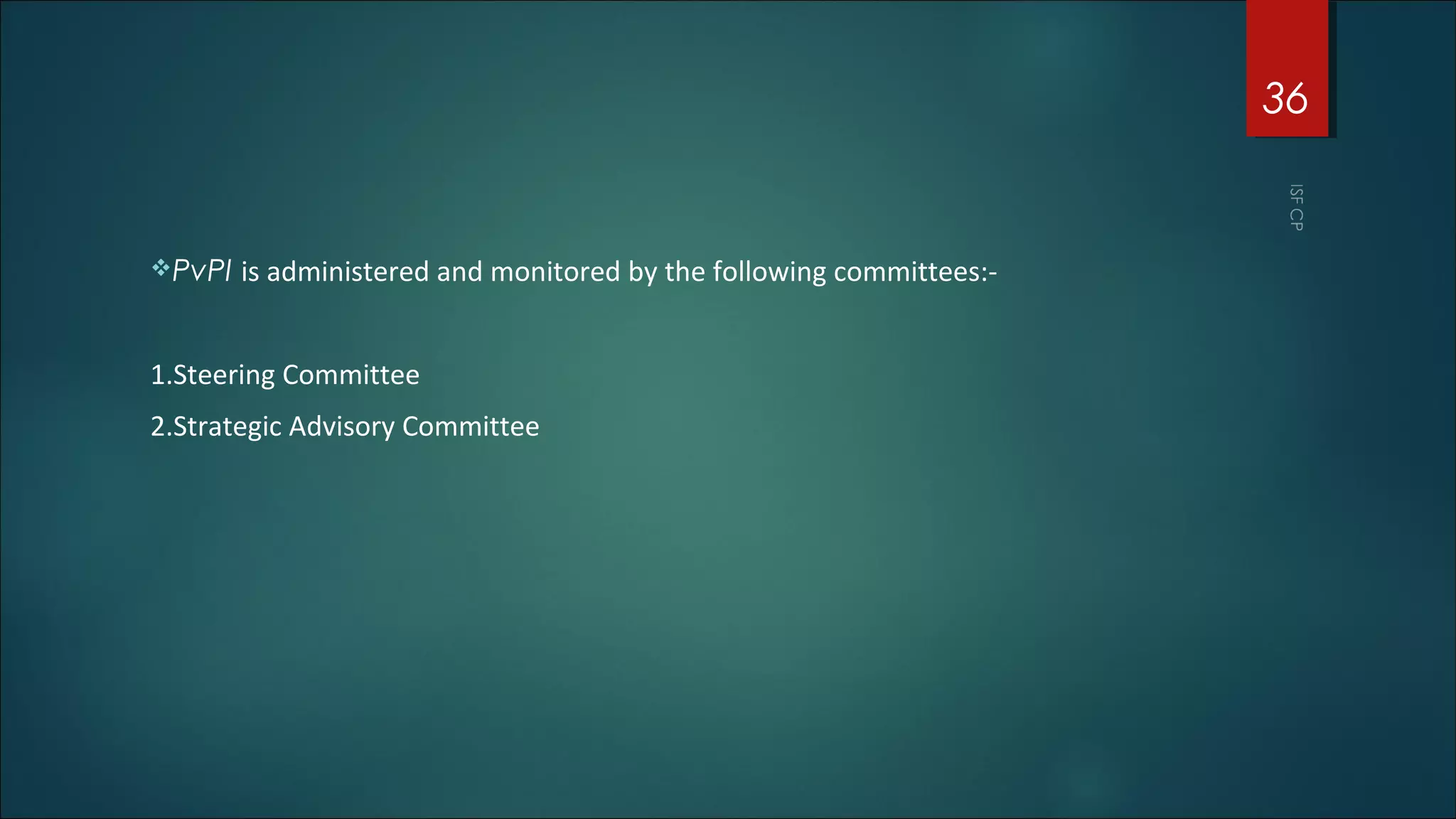
This document discusses good pharmacovigilance practices and outlines the definitions, history, and processes involved in monitoring drug safety. It highlights the methods of pharmacovigilance, including spontaneous reporting, data mining, and intensive monitoring, while detailing the evolution of adverse drug reaction (ADR) assessments over the years. The document also examines the developments in pharmacovigilance in various regions, including the USA and Europe, and the establishment of the pharmacovigilance programme in India.