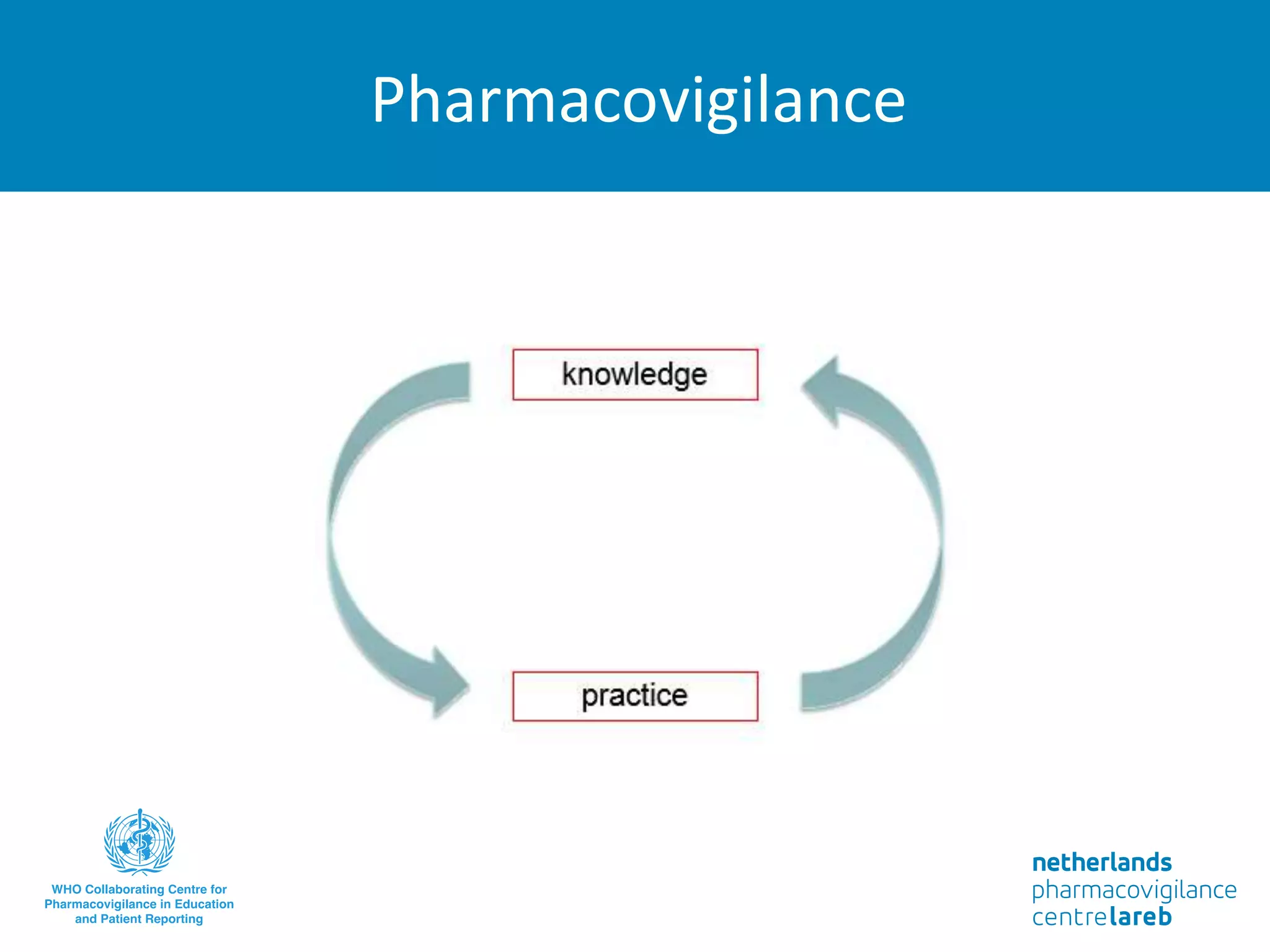
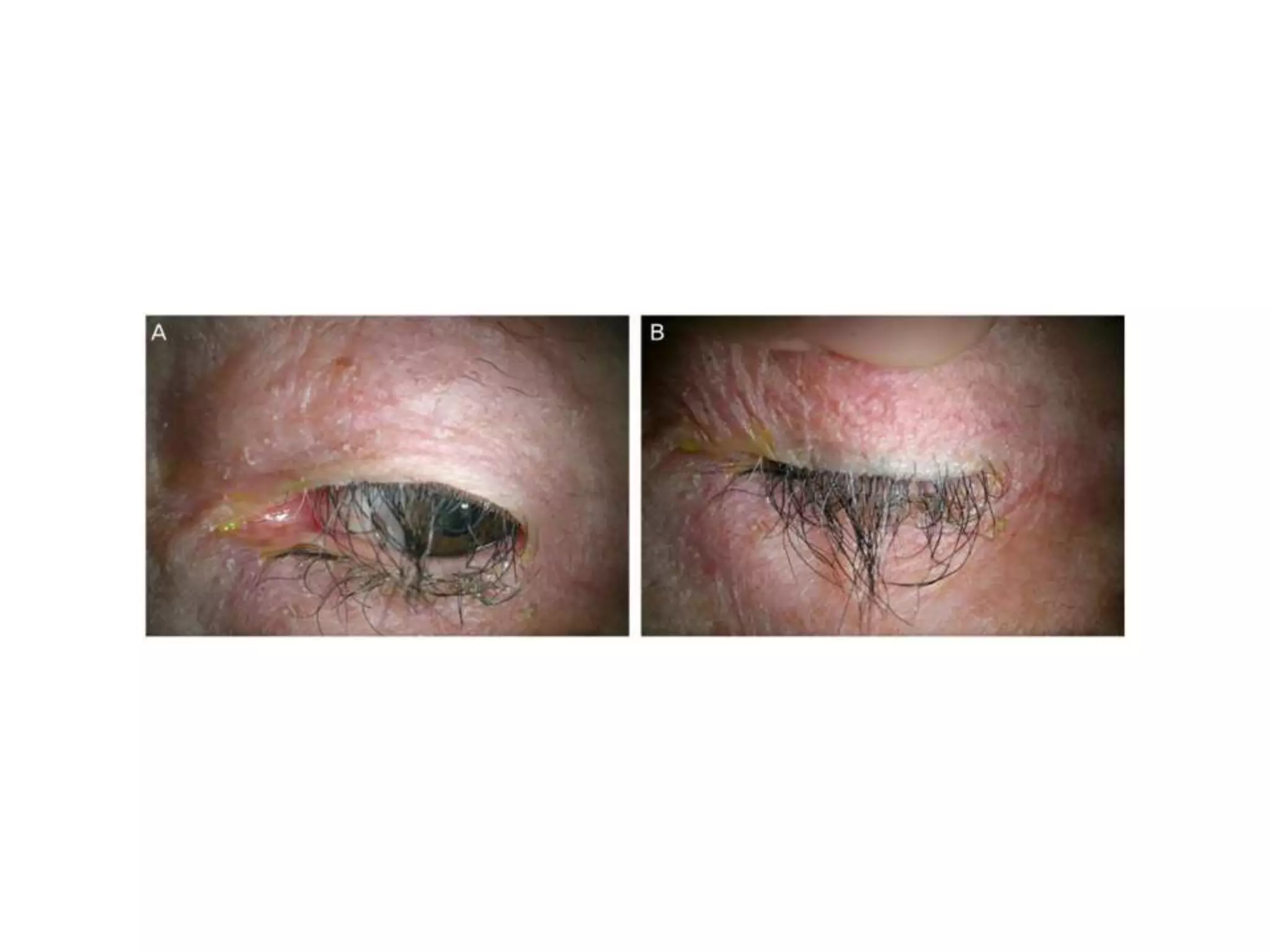
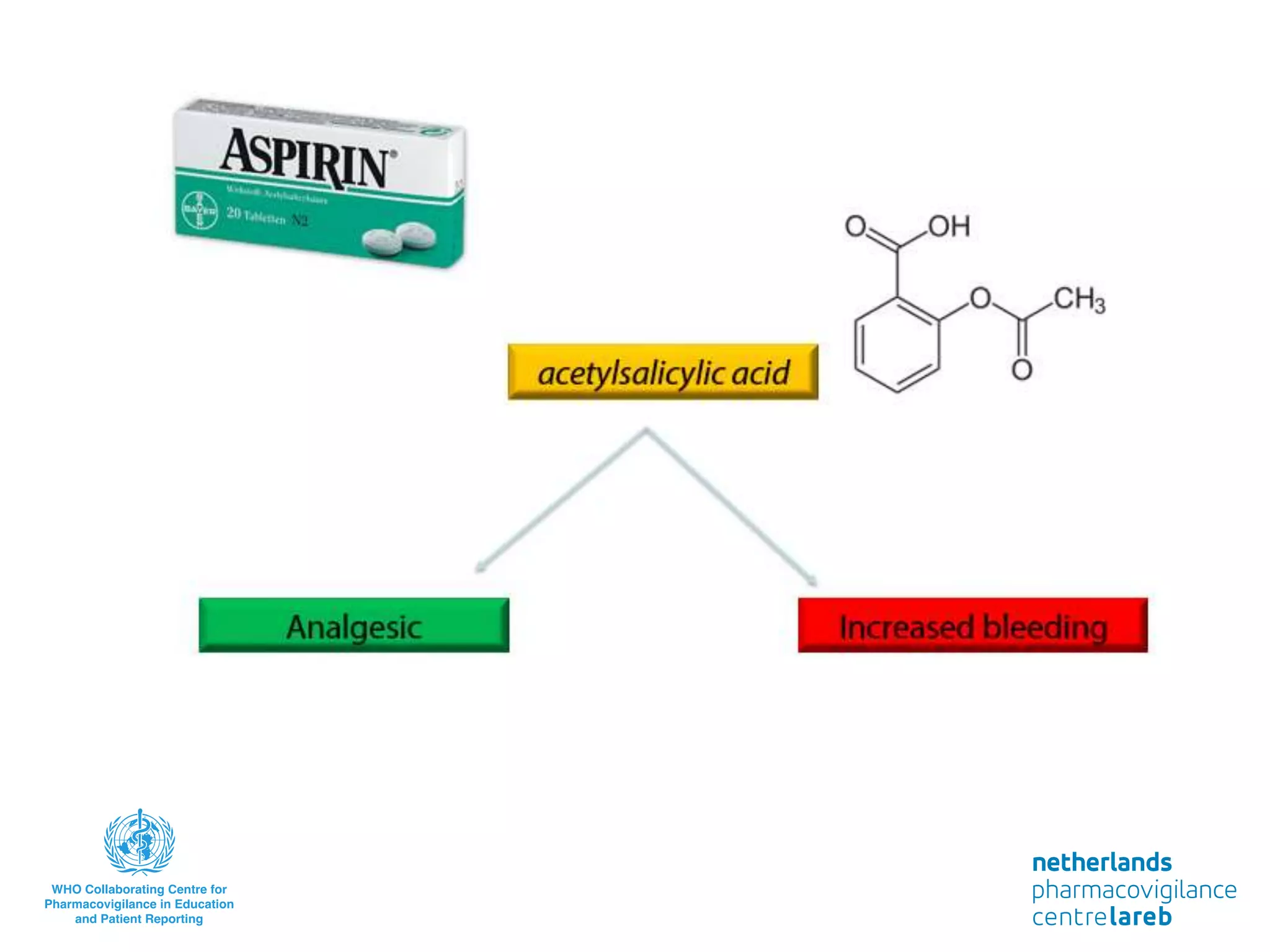
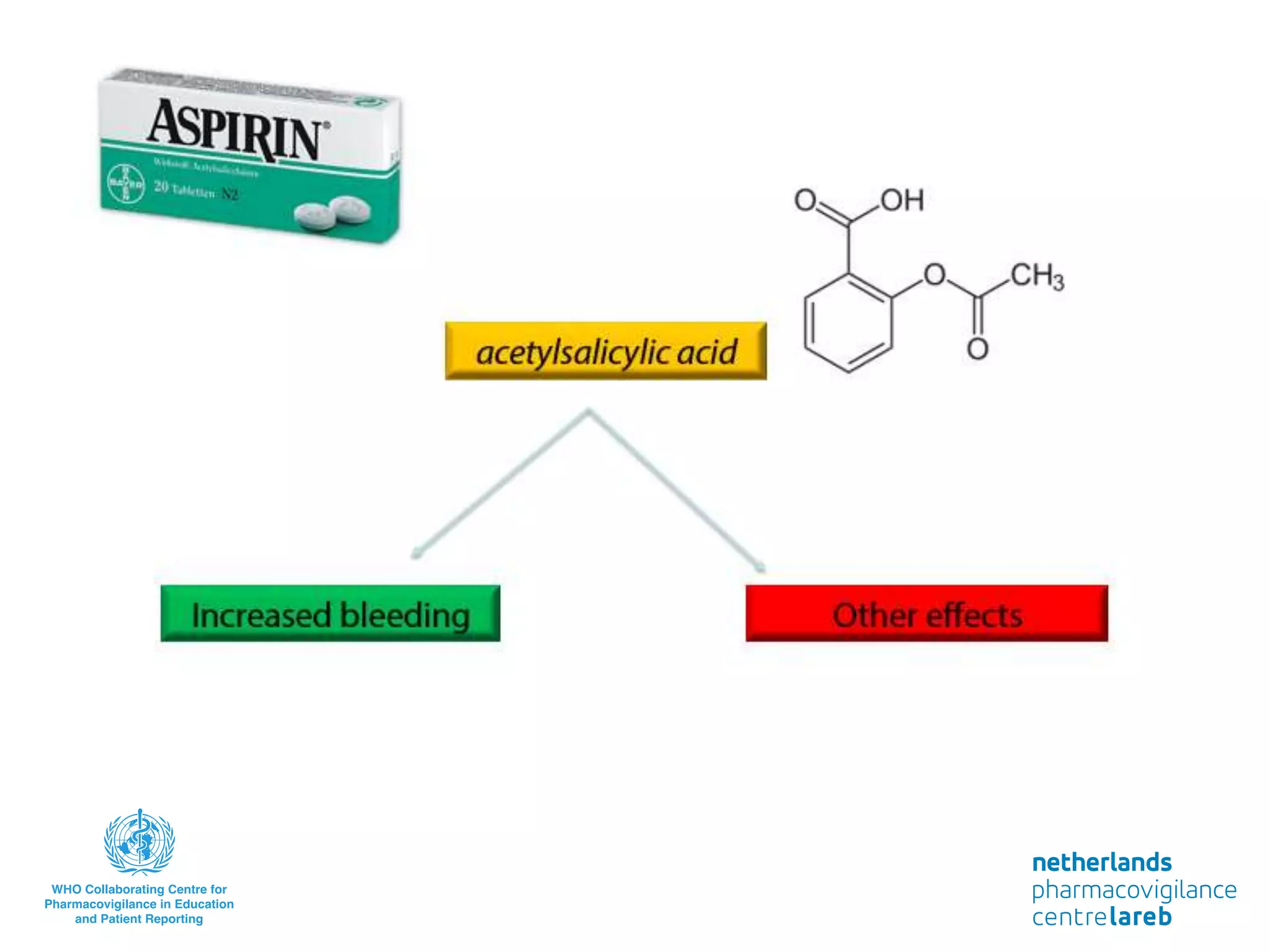
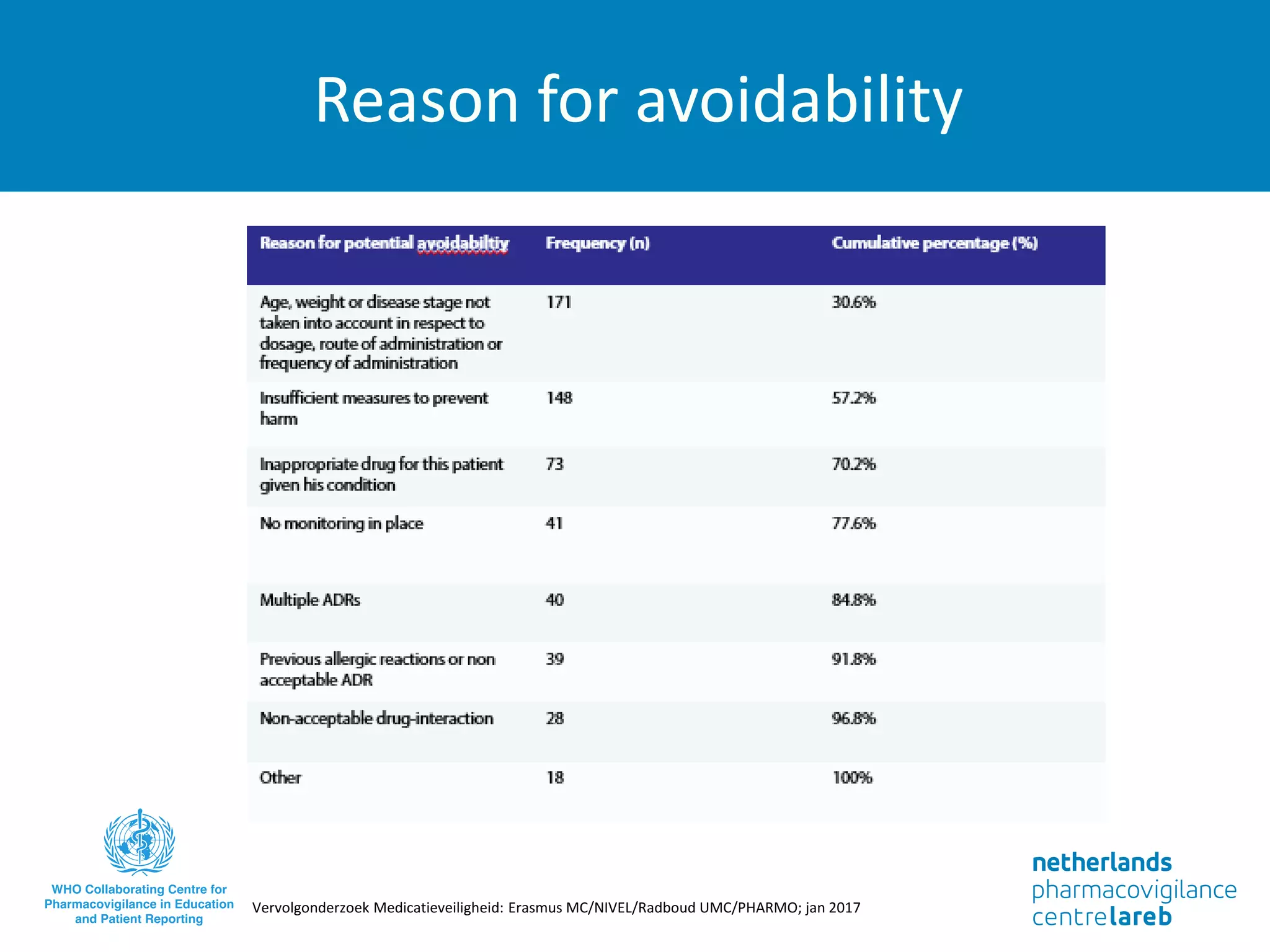
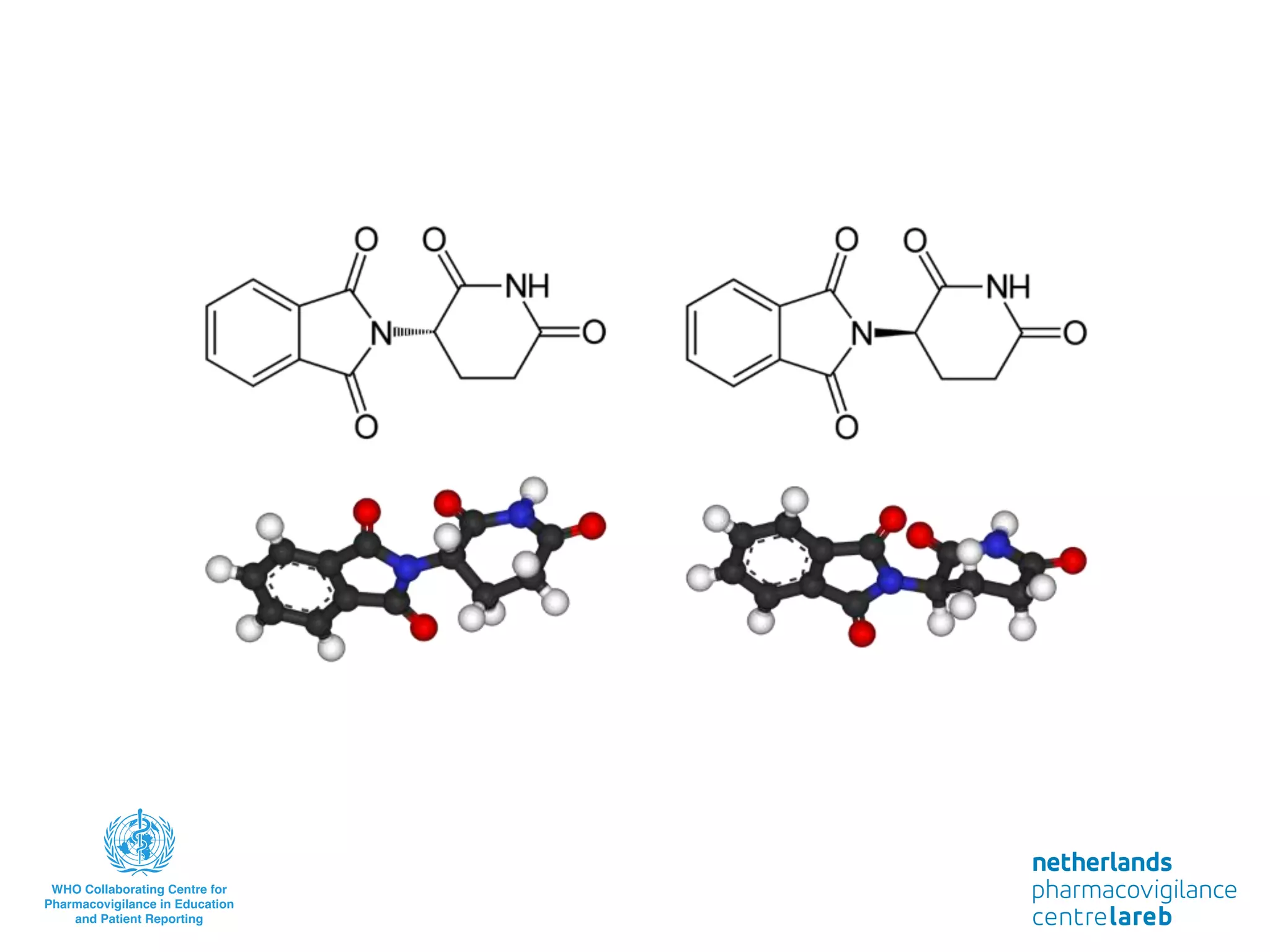
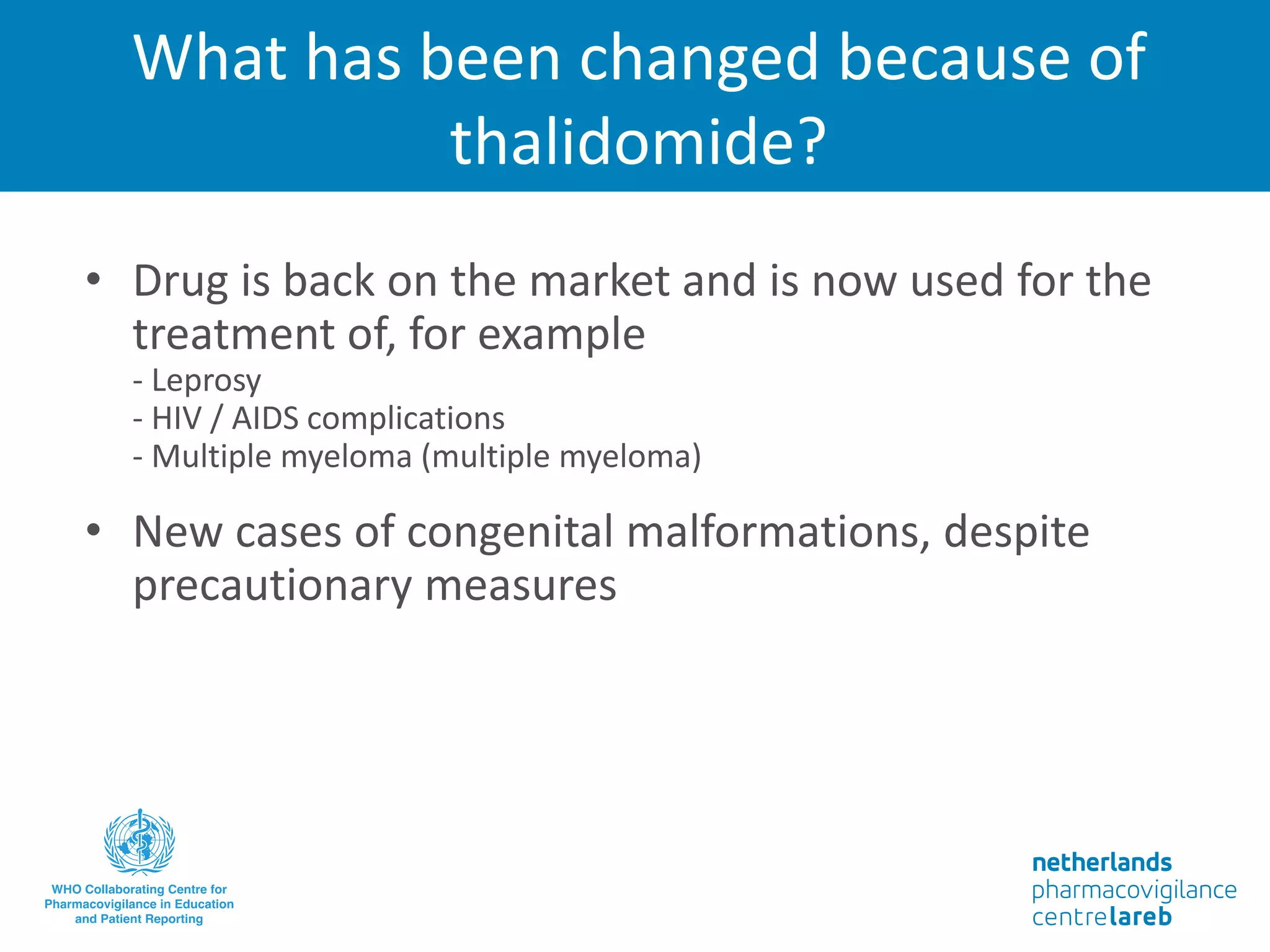
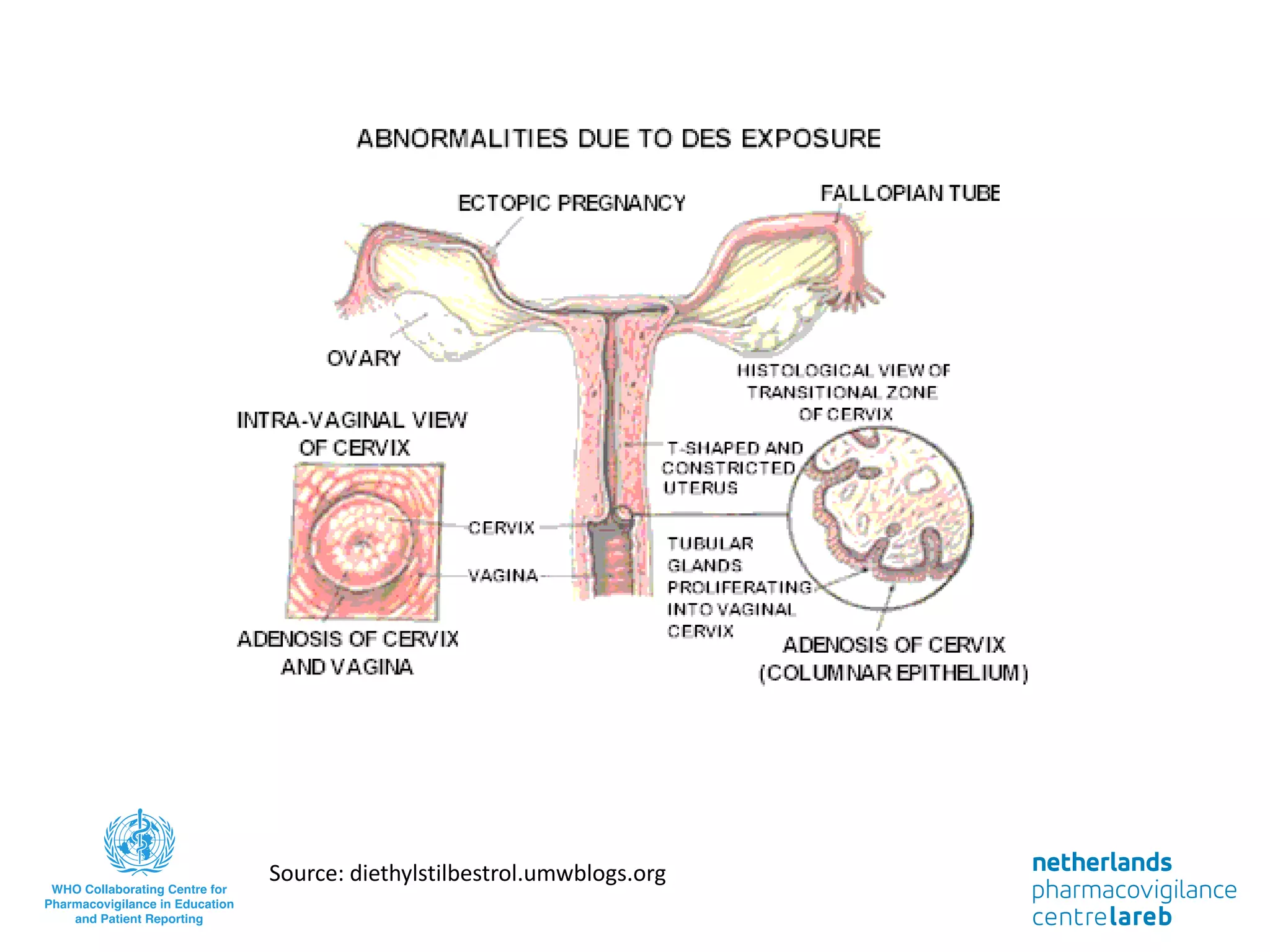
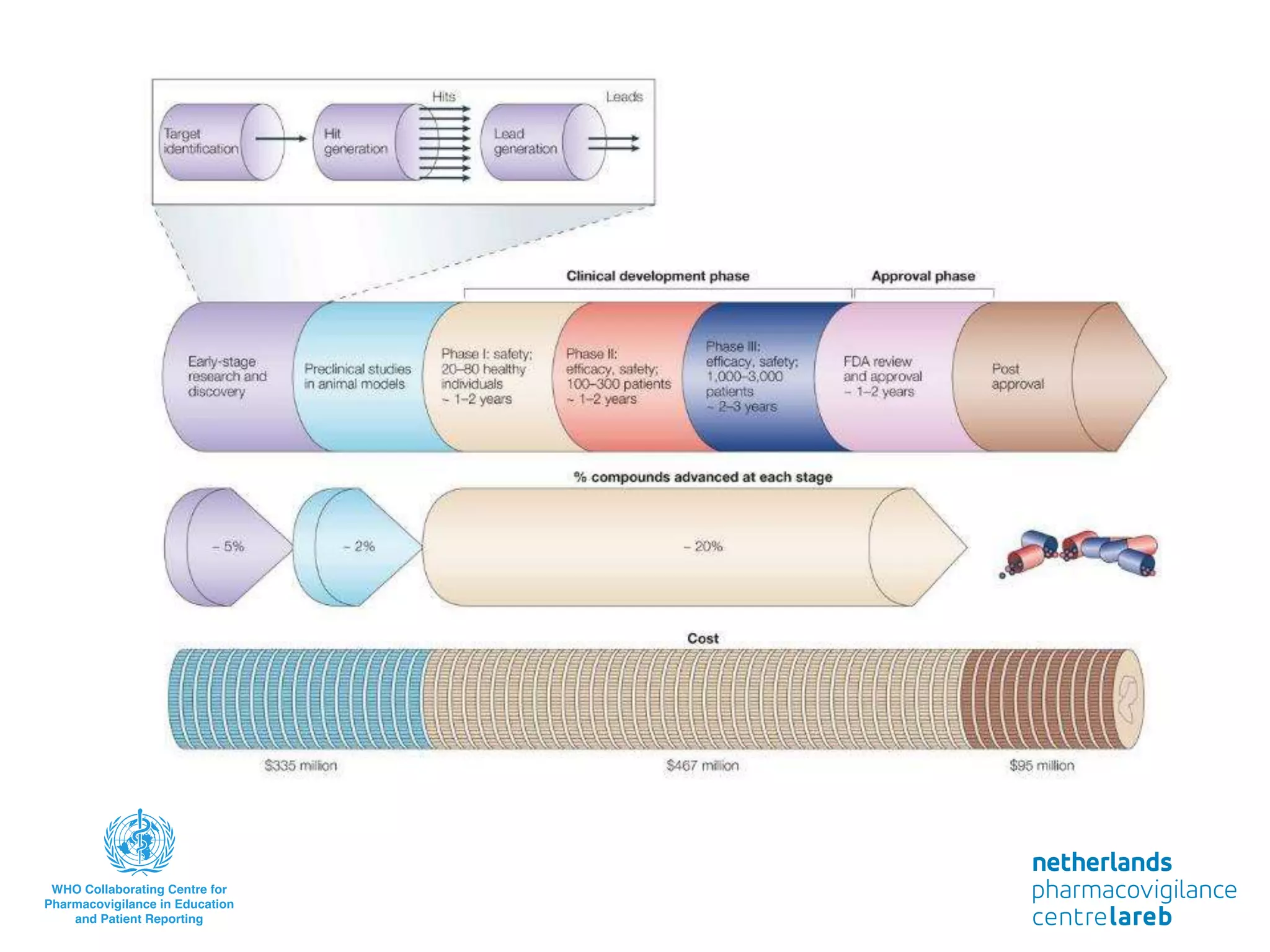
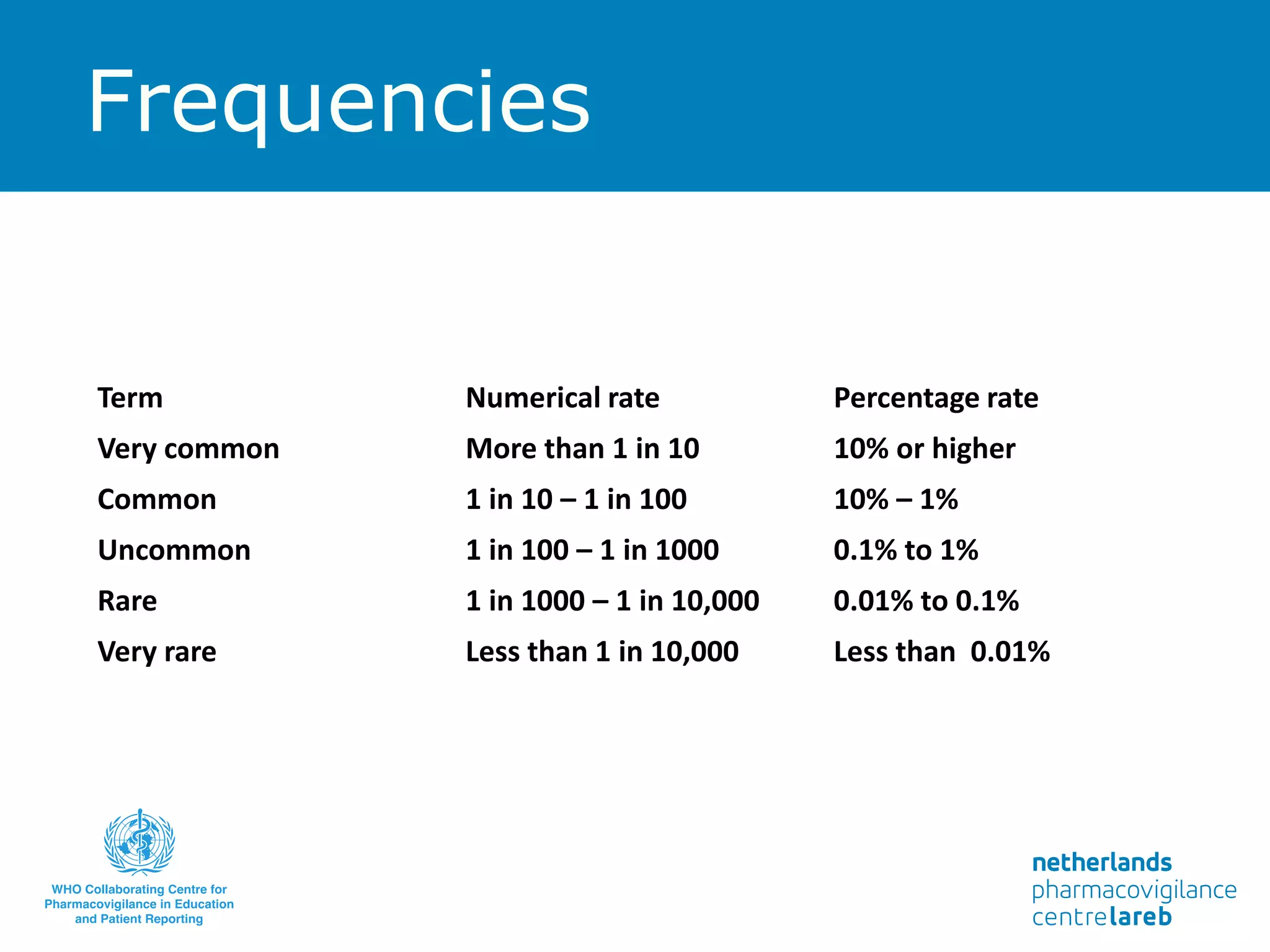
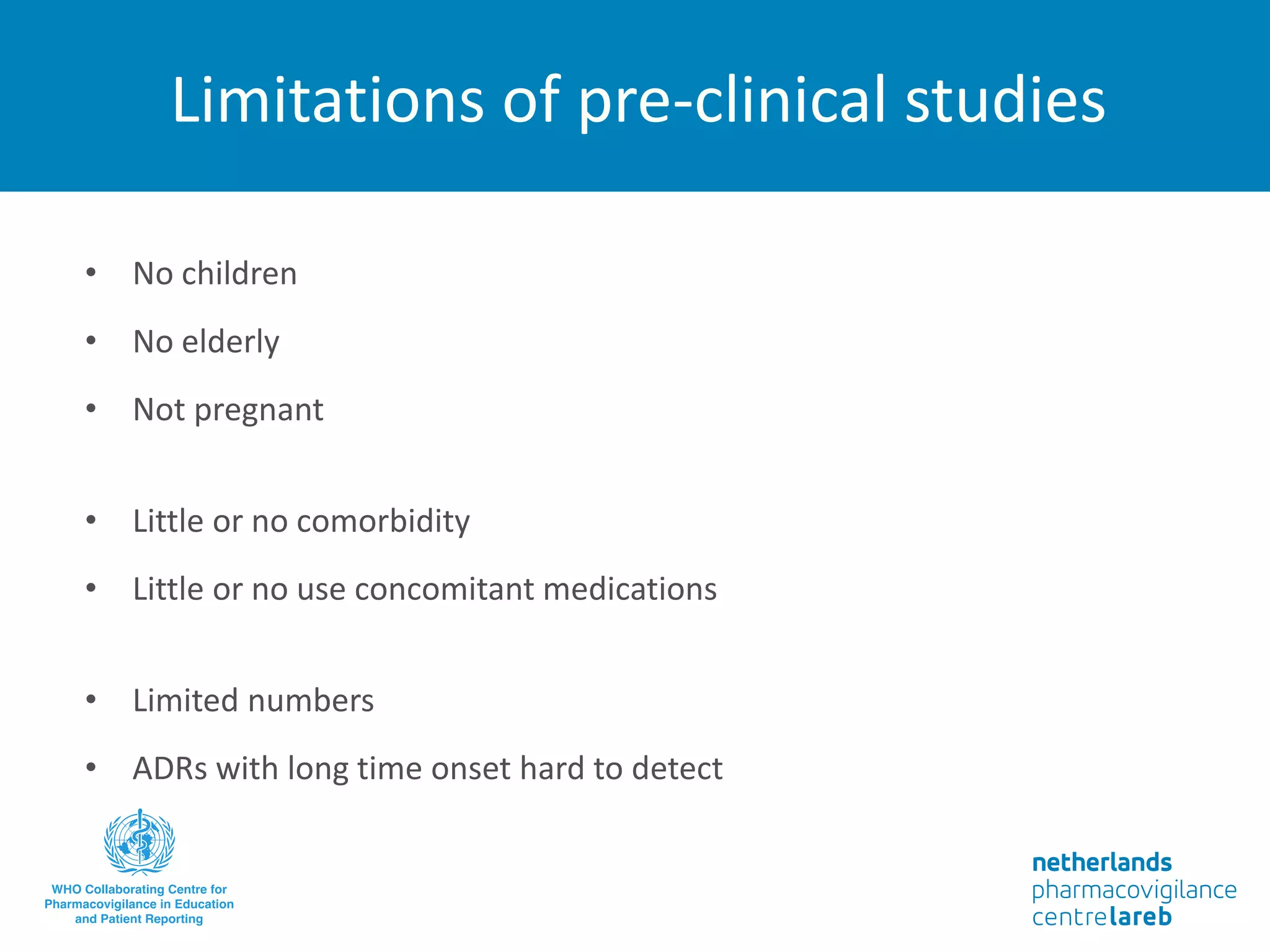
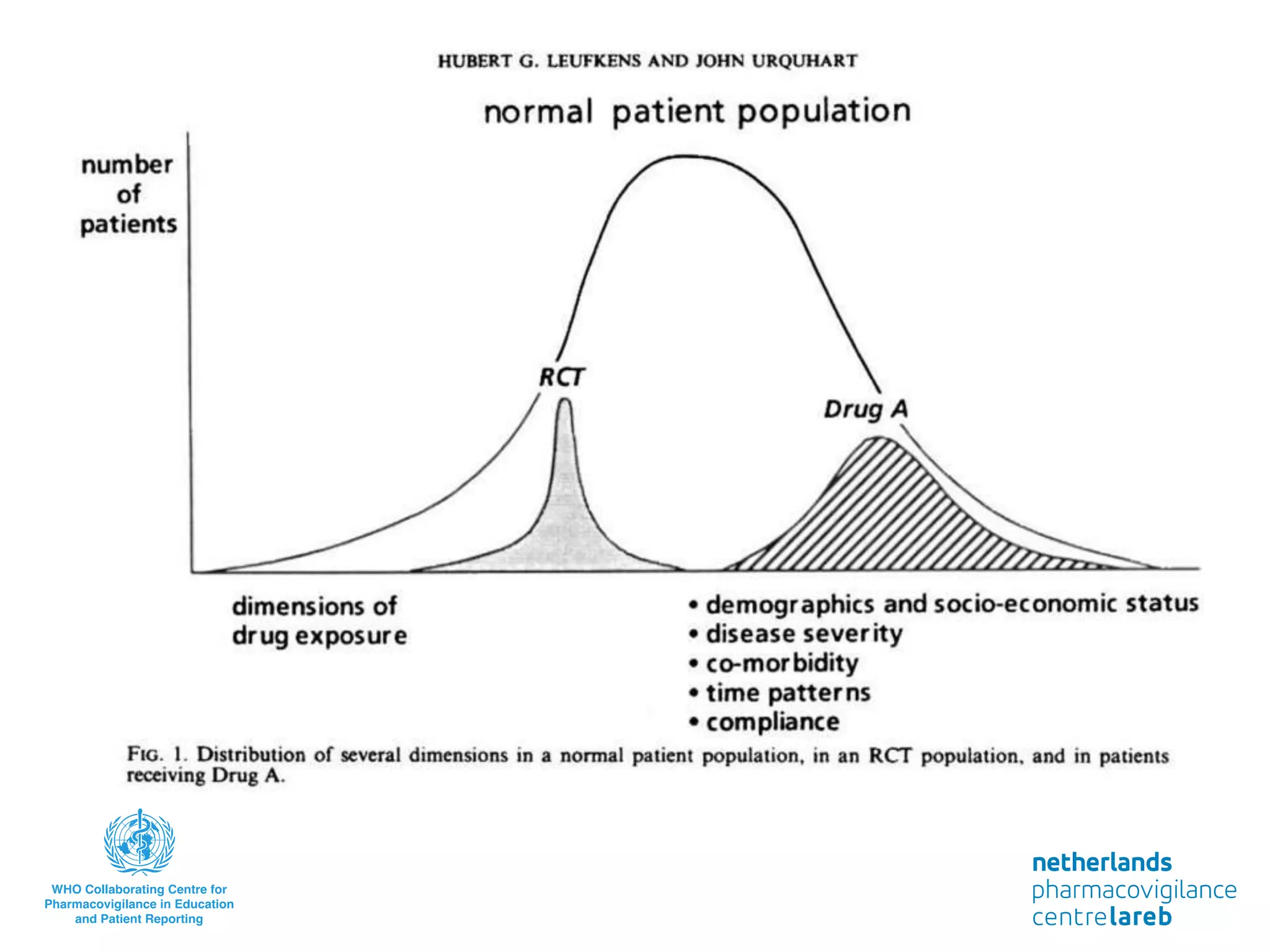
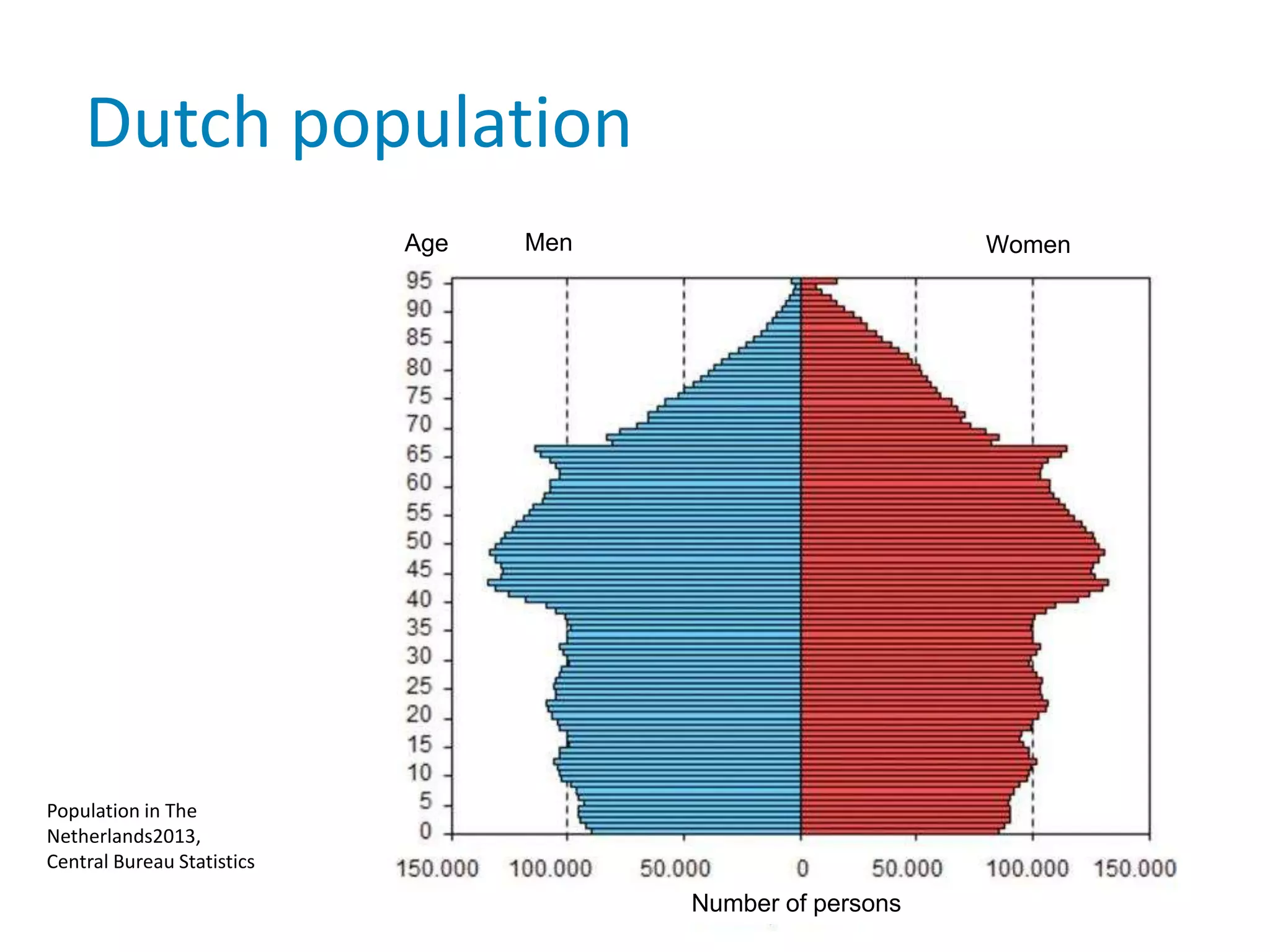
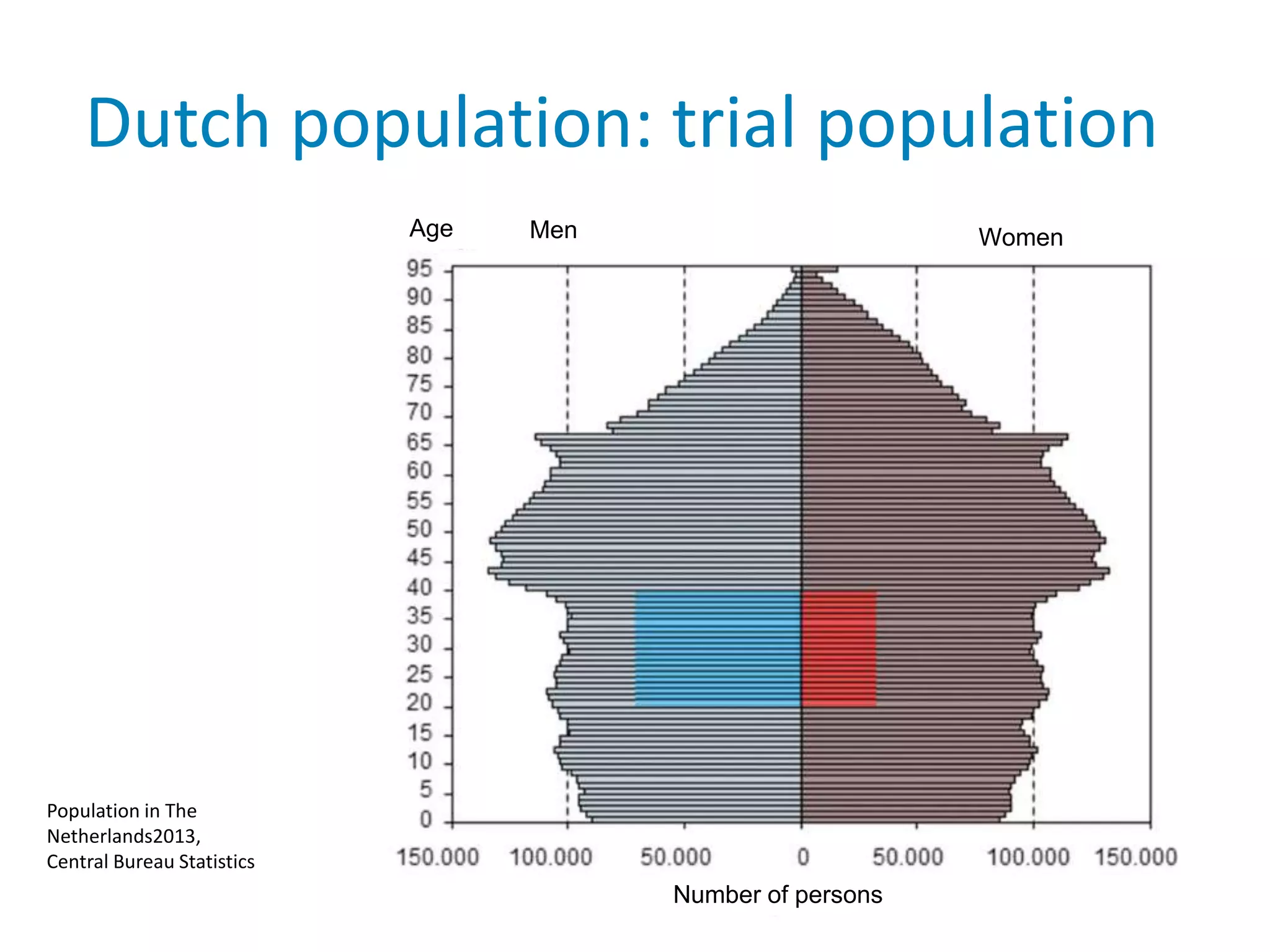
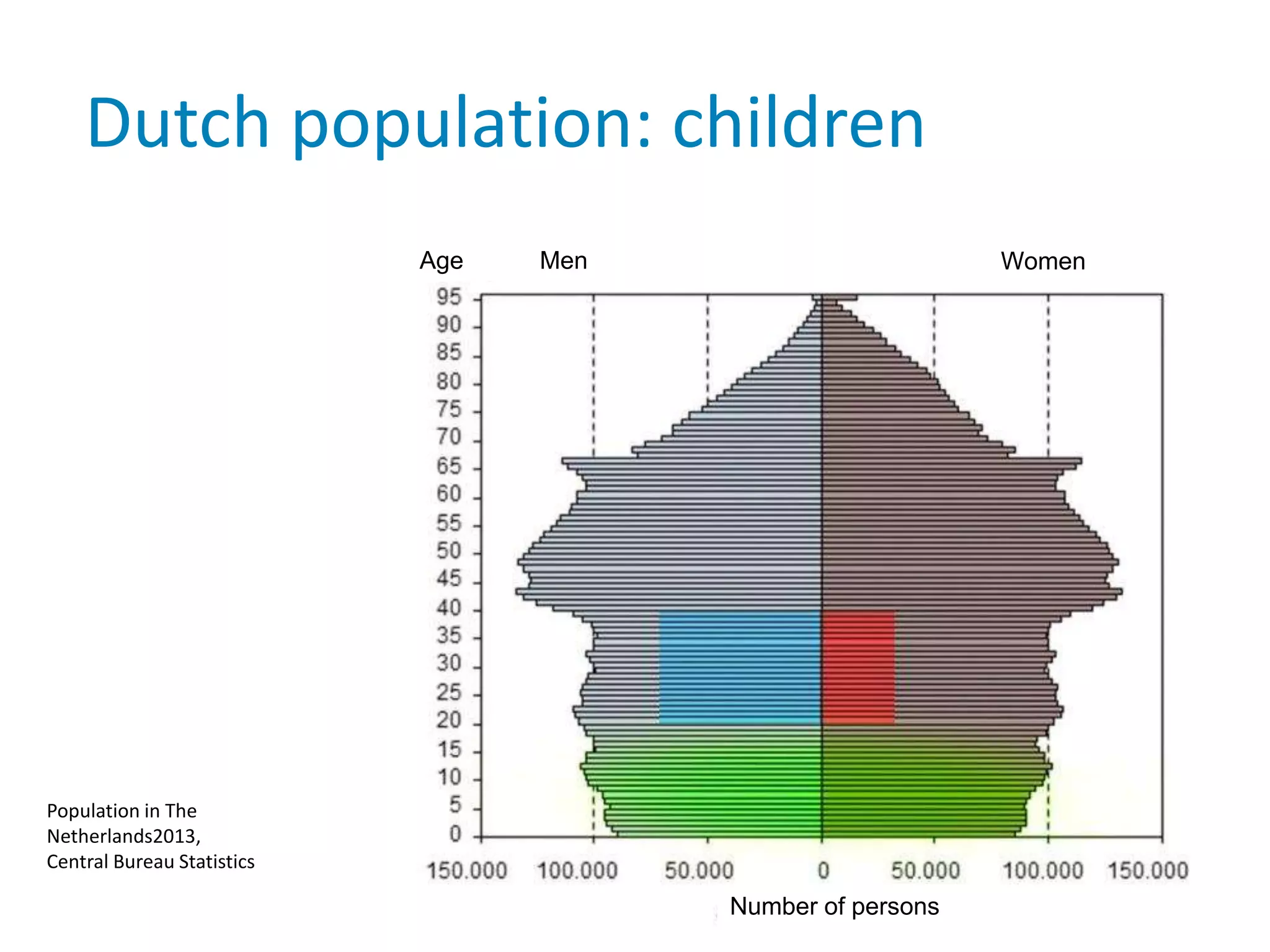
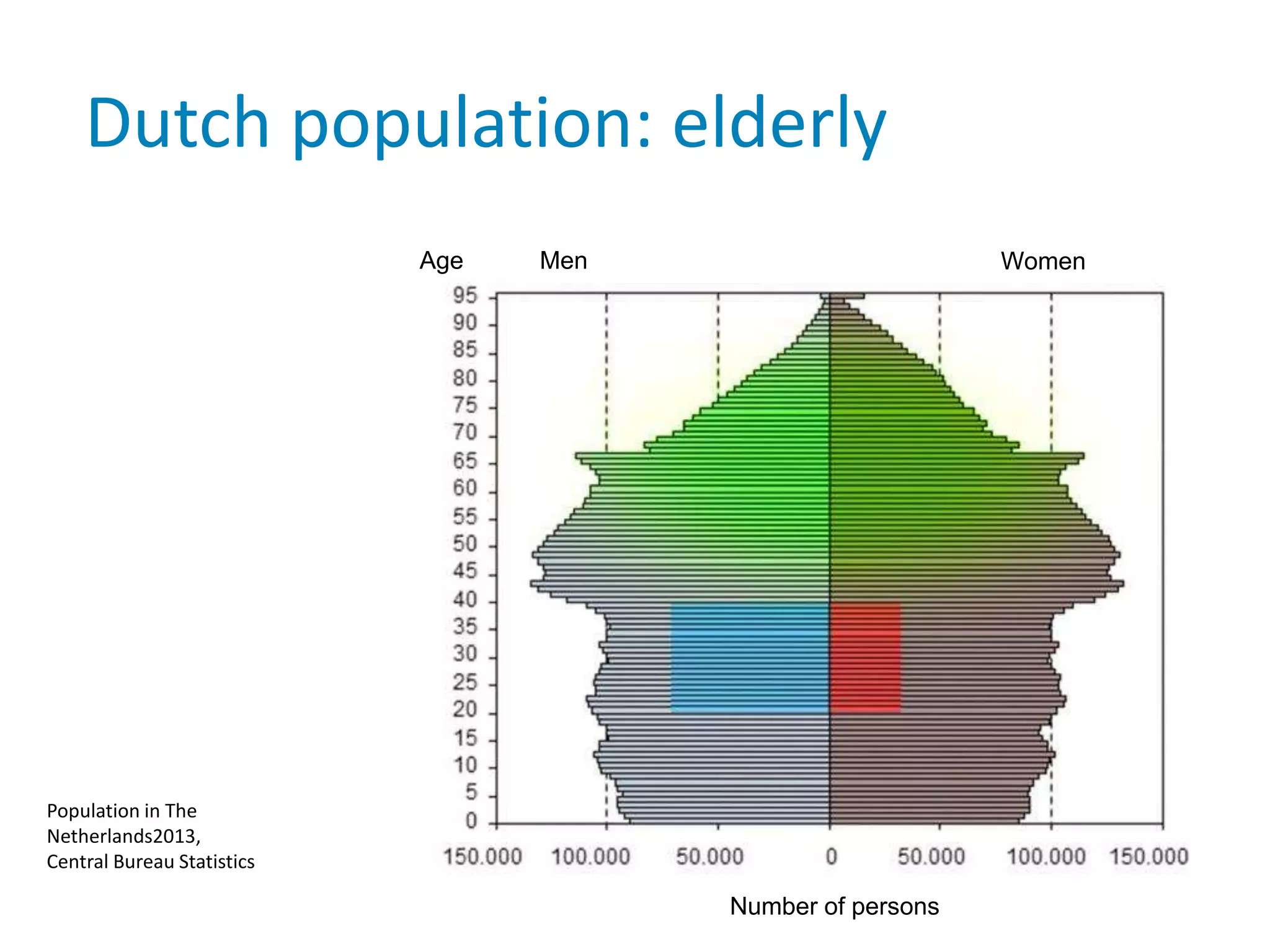
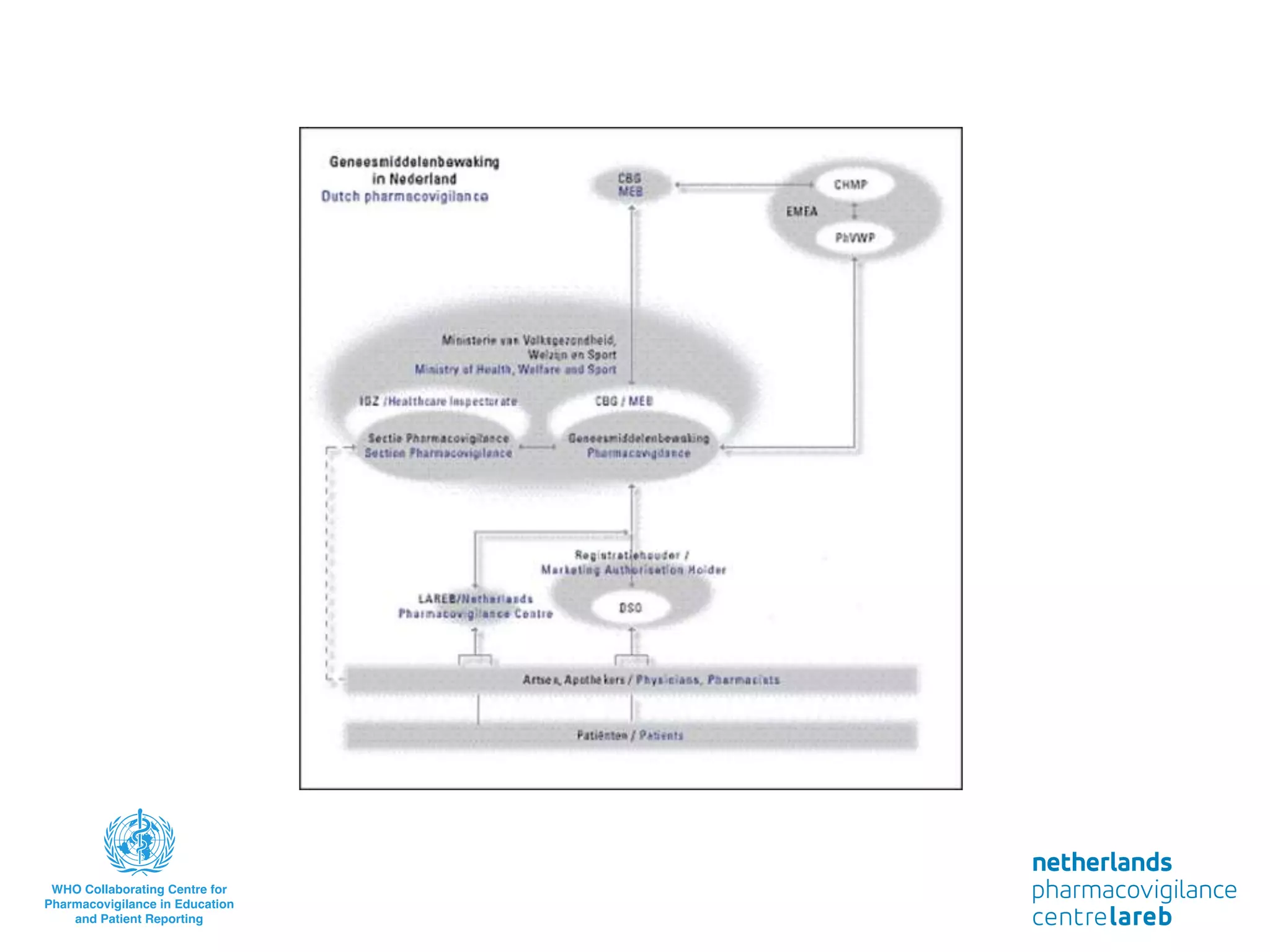
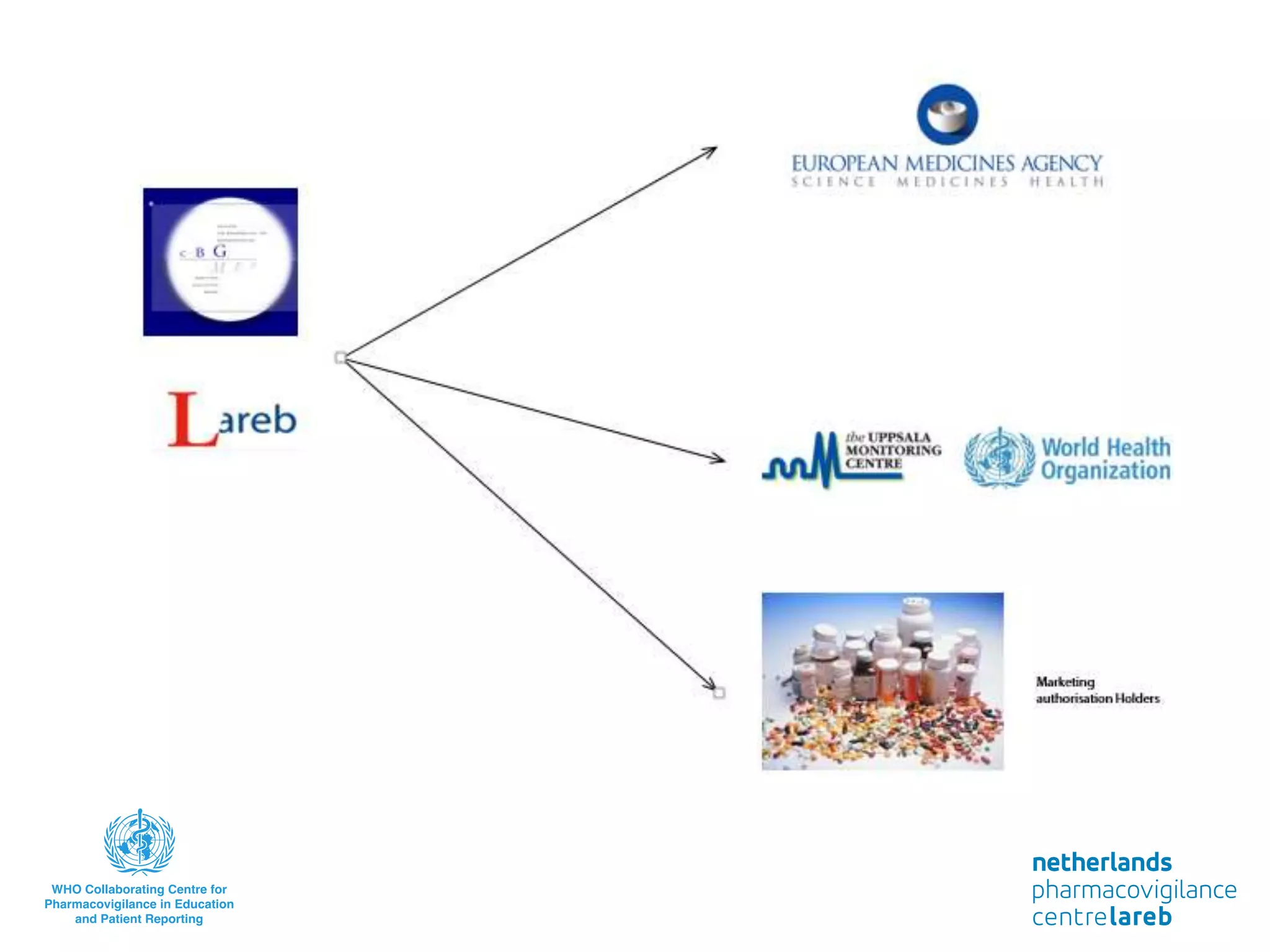
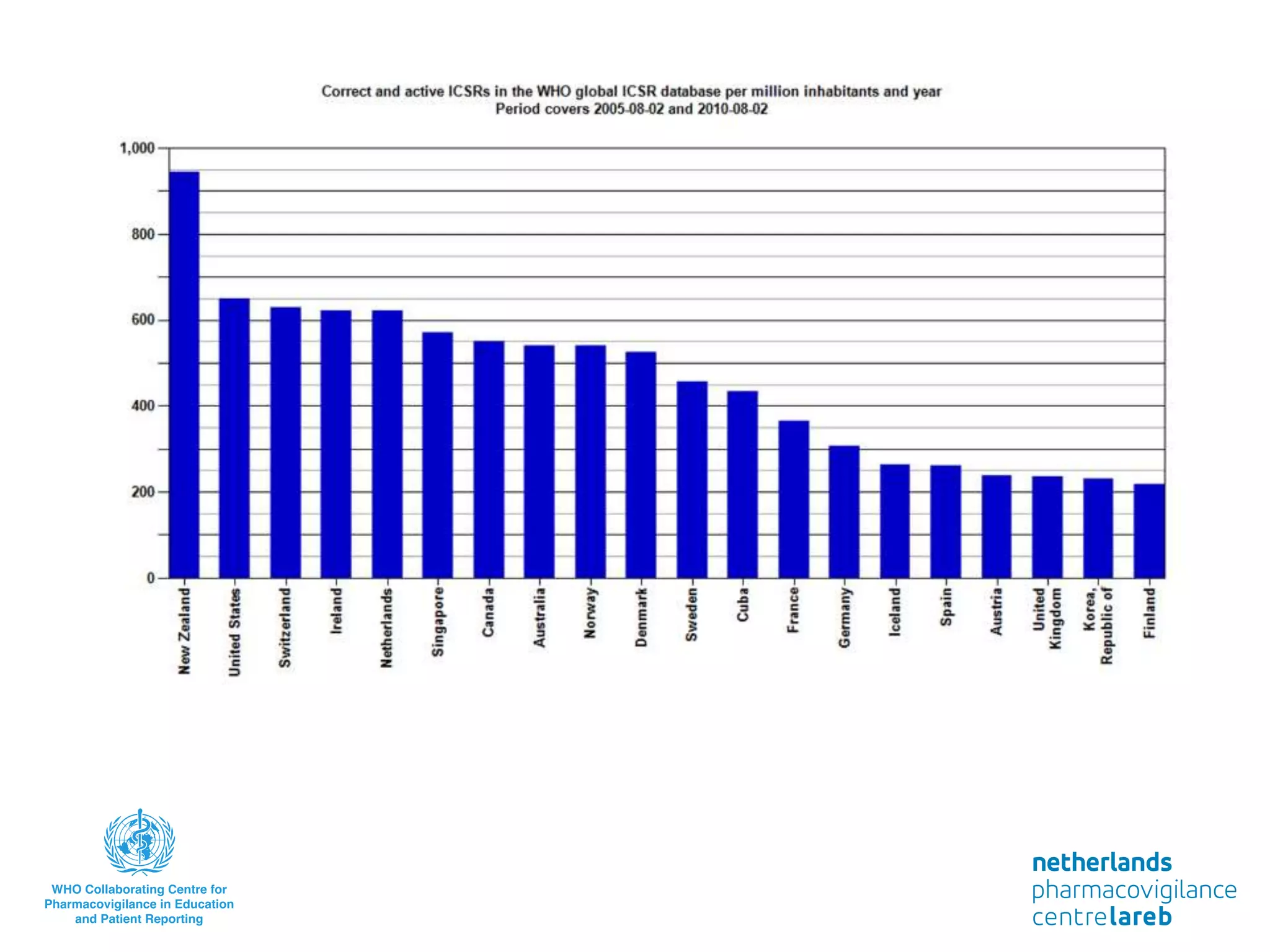
The document provides background information on a lecture about pharmacovigilance. It discusses key topics like the importance of pharmacovigilance, drug safety in practice, the need to monitor drug safety, and how pharmacovigilance is organized. The learning objectives are to understand the definition of pharmacovigilance, what constitutes a serious side effect, the history of important side effects that have influenced the field, and how drug safety is organized in the Netherlands and Europe.