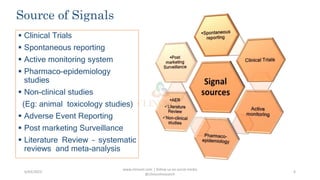
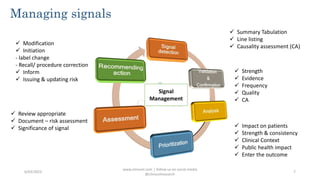
This document defines signal detection as information that suggests a new potentially causal association or aspect of a known association between an intervention and adverse or beneficial events. It discusses the importance of signal detection for identifying safety concerns, improving patient outcomes, and regulatory compliance. Several methods of signal detection are described, including data mining, quantitative statistics, adverse event monitoring, and machine learning algorithms. Challenges and future directions are also outlined.





![Methods of Signal Detection
Data Mining:
This is a technique used for identifying patterns
and links in large datasets.
Quantitative signal detection:
- Done through disproportionality statistics
- Different ways to calculate disproportionality -
Reporting Odds Ratio (ROR), Proportional
Reporting Ration (PRR) and Bayesian method
(Empirical Bayer's Geometric Mean [EBGM] &
Information Component [IC])
www.clinosol.com | follow us on social media
@clinosolresearch
8
Formulae to calculate ROR
ROR = (a/b)/(c/d)
Where,
a = no. of primary case reports on Drug X
and Adverse Event Y
b = no. of primary case report involving
Drug X and NOT Adverse Event Y
c= no. of primary case reports involving
NOT Drug X and Adverse Event Y
D= no. of primary case reports involving
NOT Drug X and NOT Adverse Event Y.
6/03/2023](https://image.slidesharecdn.com/signaldetectionpptmarch2023hannahchristina1-230505151116-703507f8/85/Signal-Detection-in-Pharmacovigilance-8-320.jpg)





