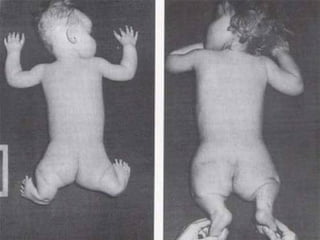
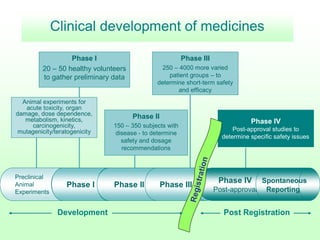
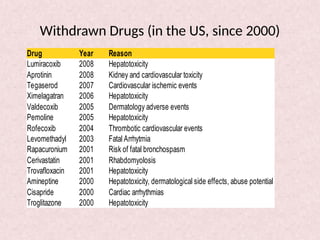
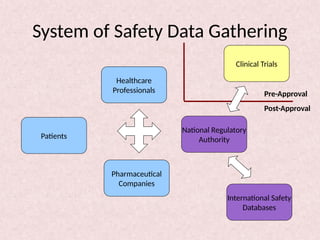
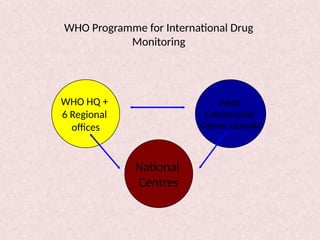
Pharmacovigilance is essential for ensuring drug safety, with India ranking second globally in population and producing over 20% of the world's generics. The document emphasizes the importance of monitoring adverse drug reactions (ADRs) to protect patients and promote safe medicine use, detailing the structure of the pharmacovigilance program in India and its objectives. It highlights the need for multidisciplinary collaboration and establishes a national pharmacovigilance program to track and analyze drug safety reports.