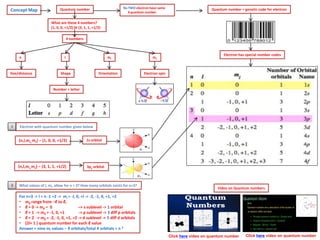
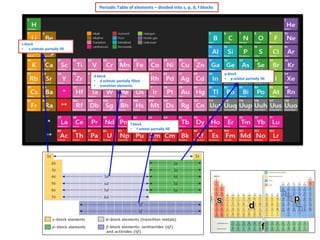
The document discusses the periodic table and electron configuration. It explains that the periodic table is divided into s, p, d and f blocks based on which atomic orbitals are being filled. It describes the patterns of electron filling according to the Aufbau principle, Hund's rule and Pauli exclusion principle. Examples of electron configurations are provided for elements in the s, p, d and f blocks to illustrate partial filling of the respective orbitals.


![Electron Notation
Atom
Positive/Negative Ion
s, p, d, f notation
Complete configuration
Noble gas notation
Condensed configuration
Noble gas notation
Complete configuration
10
Ne
1s2 2s2 2p6
10
Ne
[Ne]
10
Ne
1s2 2s2 2p6 /[Ne]
11
Na
1s2 2s2 2p6 3s1
11
Na
[Ne] 3s1
11
Na+
1s2 2s2 2p6 / [Ne]
12
Mg
1s2 2s2 2p6 3s2
12
Mg
[Ne] 3s2
12
Mg2+
1s2 2s2 2p6 / [Ne]
13
Al
1s2 2s2 2p6 3s2 3p1
13
Al
[Ne] 3s2 3p1
13
Al3+
1s2 2s2 2p6 / [Ne]
14
Si
1s2 2s2 2p6 3s2 3p2
14
Si
[Ne] 3s2 3p2
14
Si4+
1s2 2s2 2p6 / [Ne]
15
P
1s2 2s2 2p6 3s2 3p3
15
P
[Ne] 3s2 3p3
15
P3-
1s2 2s2 2p6 3s2 3p6 /[Ar]
16
S
1s2 2s2 2p6 3s2 3p4
16
S
[Ne] 3s2 3p4
16
S2-
1s2 2s2 2p6 3s2 3p6 /[Ar]
17
CI
1s2 2s2 2p6 3s2 3p5
17
CI
[Ne] 3s2 3p5
17
CI-
1s2 2s2 2p6 3s2 3p6/ [Ar]
18
Ar
1s2 2s2 2p6 3s2 3p6
18
Ar
[Ar]
19
[Ne]
18
Ar
[Ar]
K
[Ar]
4s1
19
K+
1s2 2s2 2p6 3s2 3p6 /[Ar]
20
Ca
[Ar] 4s2
20
Ca2+
1s2 2s2 2p6 3s2 3p6 / [Ar]
21
Sc
[Ar] 4s2 3d1
22
Ti
[Ar] 4s2 3d2
1s2 2s2 2p6 3s2 3p6 4s2 3d3
23
V
[Ar] 4s2 3d3
Cr
1s2 2s2 2p6 3s2 3p6 4s1 3d5
24
Cr
[Ar] 4s1 3d5
25
Mn
1s2 2s2 2p6 3s2 3p6 4s2 3d5
25
Mn
[Ar] 4s2 3d5
26
Fe
1s2 2s2 2p6 3s2 3p6 4s2 3d6
26
Fe
[Ar] 4s2 3d6
27
Co
1s2 2s2 2p6 3s2 3p6 4s2 3d7
27
Co
[Ar] 4s2 3d7
28
Ni
1s2 2s2 2p6 3s2 3p6 4s2 3d8
28
Ni
[Ar] 4s2 3d8
29
Cu
1s2 2s2 2p6 3s2 3p6 4s1 3d10
29
Cu
[Ar] 4s1 3d10
30
Zn
1s2 2s2 2p6 3s2 3p6 4s2 3d10
30
Zn
[Ar] 4s2 3d10
K
1s2
2s2
2p6
3s2
3p6 4s1
19
20
Ca
1s2 2s2 2p6 3s2 3p6 4s2
21
Sc
1s2 2s2 2p6 3s2 3p6 4s2 3d1
22
Ti
1s2 2s2 2p6 3s2 3p6 4s2 3d2
23
V
24
[Ar]](https://image.slidesharecdn.com/spdf2014pdf-140210012322-phpapp01/85/IB-Chemistry-on-Quantum-Numbers-and-Electronic-Configuration-4-320.jpg)

![s block elements
• s orbitals partially fill
1
H
He
p block elements
• p orbital partially fill
5
1s2
n = 2 period 2
B
[He] 2s2 2p1
6
1s1
2
Periodic Table – s, p, d, f blocks elements
C
[He] 2s2 2p2
7
N
[He] 2s2 2p3
3
Li
[He] 2s1
8
O
[He] 2s2 2p4
4
Be
[He] 2s2
9
F
[He] 2s2 2p5
11
Na
[Ne] 3s1
10
Ne
[He] 2s2 2p6
12
Mg
[Ne] 3s2
13
Al
[Ne] 3s2 3p1
14
20
K
Ca
[Ne] 3s2 3p2
[Ar]
15
P
[Ne] 3s2 3p3
[Ar]
4s2
16
S
[Ne] 3s2 3p4
17
19
Si
4s1
CI
[Ne] 3s2 3p5
18
Ar
[Ne] 3s2 3p6
d block elements
• d orbitals partially fill
• transition elements
21
Sc
[Ar] 4s2 3d1
22
Ti
[Ar] 4s2 3d2
23
V
[Ar] 4s2 3d13
24
Cr
[Ar] 4s1 3d5
25
Mn
[Ar] 4s2 3d5
26
Fe
[Ar] 4s2 3d6
27
Co
[Ar] 4s2 3d7
28
Ni
[Ar] 4s2 3d8
29
Cu
[Ar] 4s1 3d10
30
Zn
[Ar] 4s2 3d10
f block elements
• f orbitals partially fill
Video on electron configuration
Click here electron structure
Click here video on s,p,d,f notation
Click here video s,p,d,f blocks,](https://image.slidesharecdn.com/spdf2014pdf-140210012322-phpapp01/85/IB-Chemistry-on-Quantum-Numbers-and-Electronic-Configuration-6-320.jpg)
![Periodic Table – s, p, d, f blocks elements
Electron structure
Chromium d block (Period 4)
1s2 2s2 2p6 3s2 3p6 4s1 3d5
[Ar] 4s1 3d5
d block – d partially filled
Electron structure
Cadmium d block (Period 5)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10
[Kr] 5s2 4d10
d block – d partially filled
Electron structure
Germanium p block, Gp 4 (Period 4)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
[Ar] 4s2 3d10 4p2
Gp 4 -4 valence electron
Electron structure
Mercury d block (Period 6)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p6 6s2 4f14 5d10
[Xe] 6s2 4f14 5d10
d block – d partially filled
Electron structure
Iodine p block, Gp 7 (Period 5)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p5
[Kr] 5s2 4d10 5p5
Gp 7 - 7 valence electron
Electron structure
Lead p block, Gp 4 (Period 6)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p6 6s2 4f14 5d106p2
[Xe] 6s2 4f14 5d10 6p2
Gp 4 -4 valence electron](https://image.slidesharecdn.com/spdf2014pdf-140210012322-phpapp01/85/IB-Chemistry-on-Quantum-Numbers-and-Electronic-Configuration-7-320.jpg)
![Periodic Table – s, p, d, f blocks elements
s block elements
• s orbitals partially fill
1
H
He
5
1s2
n = 2 period 2
B
[He] 2s2 2p1
6
1s1
2
p block elements
• p orbital partially fill
C
[He] 2s2 2p2
7
N
[He] 2s2 2p3
3
Li
[He] 2s1
8
O
[He] 2s2 2p4
4
Be
[He] 2s2
9
F
[He] 2s2 2p5
11
Na
[Ne] 3s1
10
Ne
[He] 2s2 2p6
12
Mg
[Ne] 3s2
13
Al
[Ne] 3s2 3p1
14
Si
[Ne] 3s2 3p2
15
P
[Ne] 3s2 3p3
16
S
[Ne] 3s2 3p4
17
CI
[Ne] 3s2 3p5
18
Ar
[Ne] 3s2 3p6
19
K
20
1
Ca
[Ar]
[Ar]
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p6 6s2 4f14 5d106p2
[Xe] 6s2 4f14 5d10 6p2
4s1
4s2
Identify position elements P, Q, R, S and T
Electron configuration :
P – 3s2 3p6
Q – 4s2 4p5
R – 3s2 3p6 4s2
S – 1s2 2s2 2p6 3s2 3p6 3d3 4s2
T – 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6
Answer
2
Write electron configuration for X, Y and Z
Element
Group
Period
X
2
3
Y
15
2
Z
18
3
Answer
Element
Group
Period
Classification
P
8/18
3
Noble gas
Q
7/17
4
p block
R
2
4
s block
S
5
4
d block
T
8/18
4
Noble gas
X – 1s2 2s2 2p6 3s2
Y – 1s2 2s2 2p3
Z – 1s2 2s2 2p6 3s2 3p6
3
Write electron structure for ions:
•
•
•
•
•
•
O - 1s2 2s2 2p4
O2- V - 1s2 2s2 2p6 3s2 3p6 4s2 3d3
V3+ Cu - 1s2 2s2 2p6 3s2 3p6 4s2 3d9
Cu2+ -
Answer
Write electron structure for ions:
•
•
•
•
•
•
O - 1s2 2s2 2p4
O2- -1s2 2s2 2p6
V - 1s2 2s2 2p6 3s2 3p6 4s2 3d3
V 3+ - 1s2 2s2 2p6 3s2 3p6 4s0 3d2
Cu - 1s2 2s2 2p6 3s2 3p6 4s2 3d9
Cu 2+ - 1s2 2s2 2p6 3s23p6 4s0 3d9](https://image.slidesharecdn.com/spdf2014pdf-140210012322-phpapp01/85/IB-Chemistry-on-Quantum-Numbers-and-Electronic-Configuration-8-320.jpg)