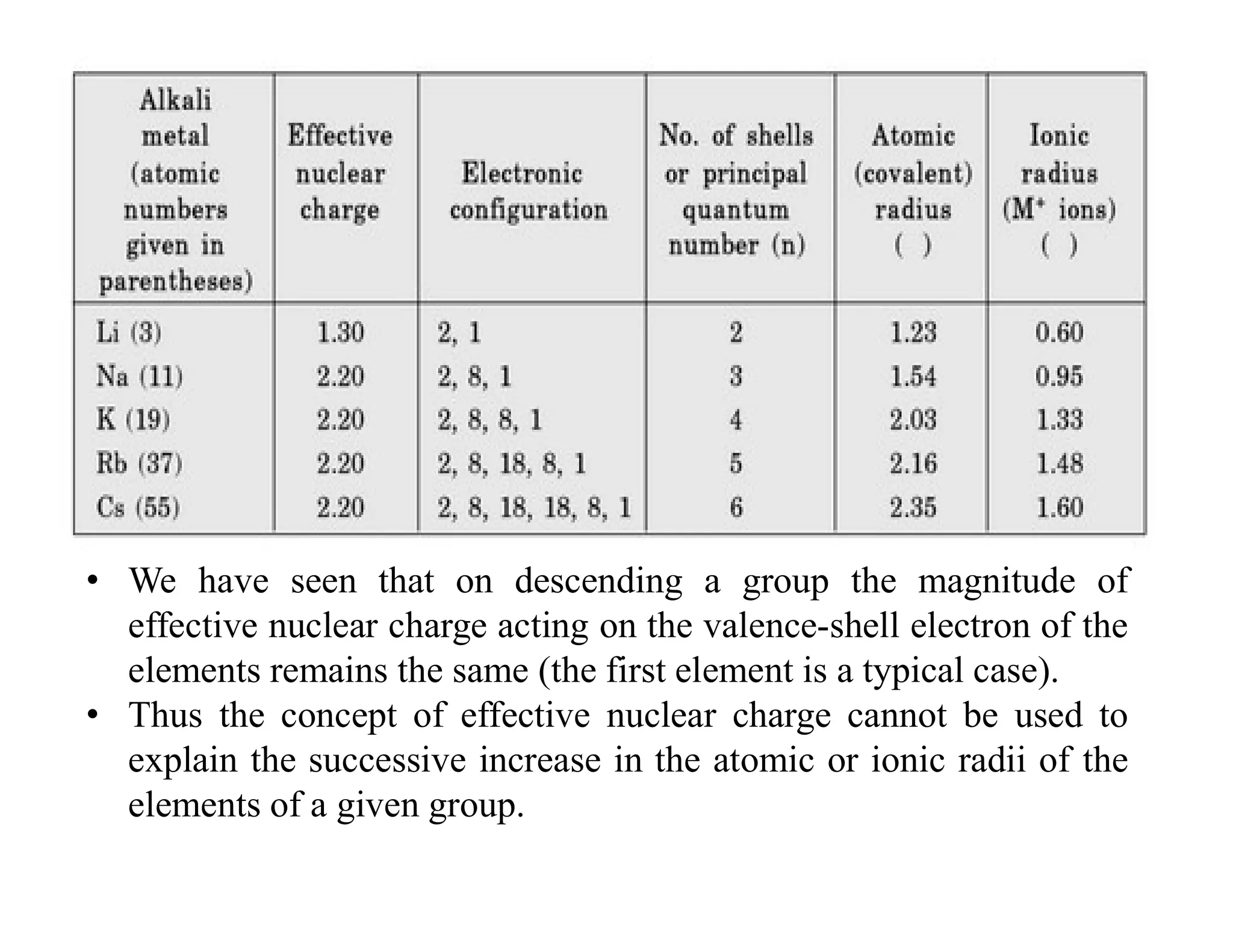
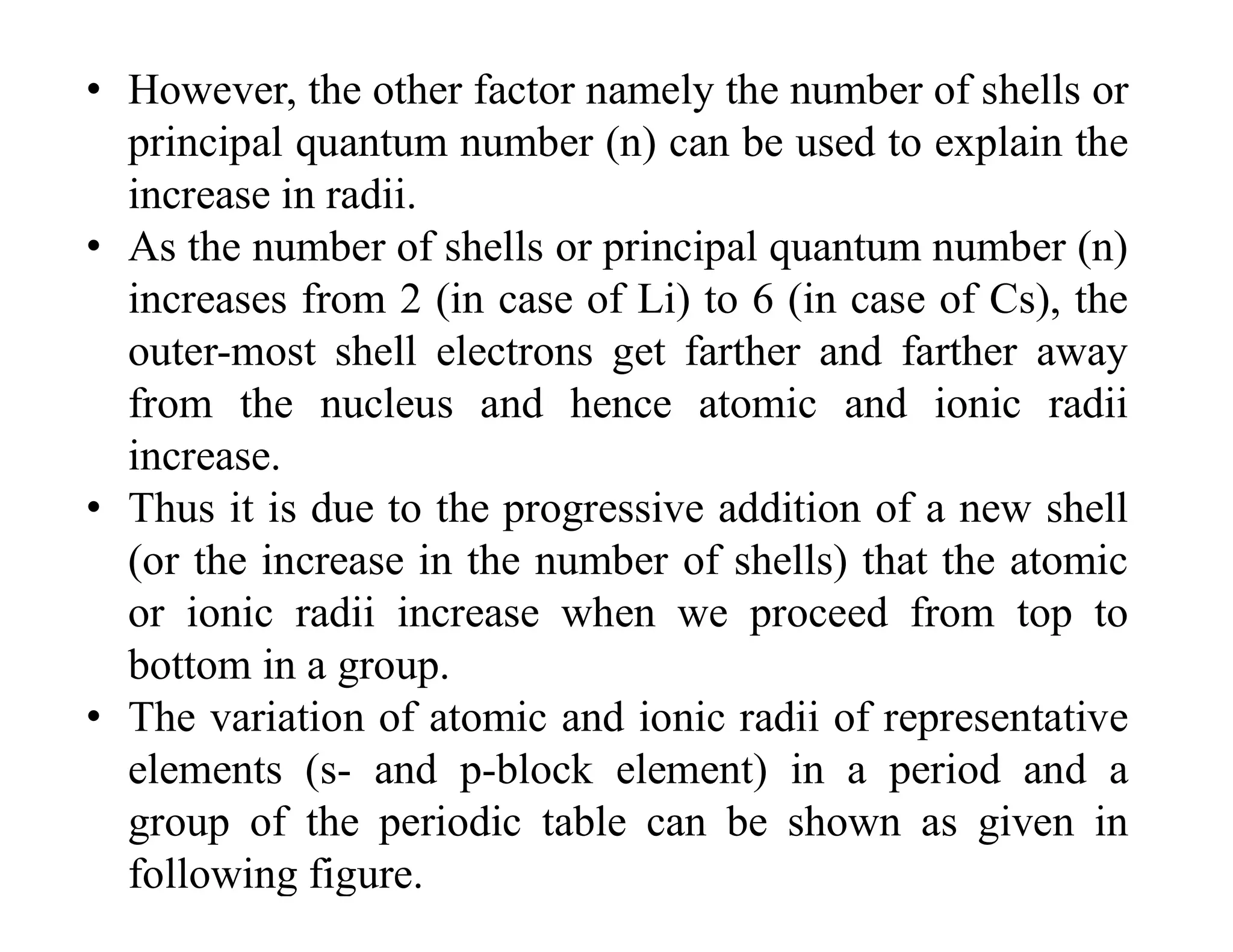
The document discusses the concept of effective nuclear charge. It explains that the actual charge experienced by valence electrons is less than the true nuclear charge due to shielding by inner electrons. This decreased charge is called the effective nuclear charge (Zeff). Slater's rules provide a method to calculate the screening constant σ and thus determine Zeff. The concept of Zeff is applied to explain trends in ionization energy, filling of electron shells, and properties of cations, anions, and across the periodic table.





















![• Why 4s orbital is filled in preference to 3d orbitals can be
explained as follows:
• The two configurations that are theoretically possible for
K-atom are:
• The calculated value of Zeff experienced by 4s1 electron of
K-atom [configuration (a)] equal to 2.20.
• The value of Zeff experienced by 3d electron of K-atom
[configuration (b)] can be calculated as follows:
– σ for 3d electron in structure (b) = 0.35 x 0 + 1.0 x 18 = 18
– Zeff experienced by 3d electron = 19 – 18 = 1.0
4s1 → Zeff → 2.20
3d1 → Zeff → 1.0](https://image.slidesharecdn.com/effectiveatomicnumberean-201223015428/75/Effective-Atomic-Number-EAN-22-2048.jpg)