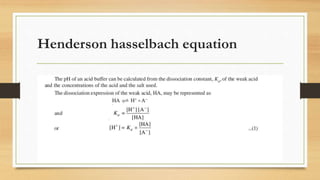
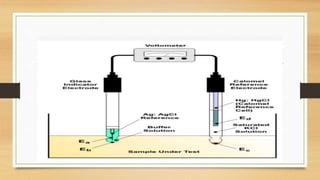
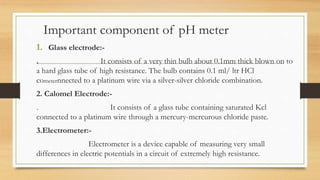
The document discusses buffer systems, important for maintaining stable pH in laboratory and industrial processes, and describes various types of buffers, including acidic and basic buffers. It includes explanations of the Henderson-Hasselbalch equation, the Sorensen pH scale, and methods for pH determination, emphasizing the electrometric method using pH meters as reliable tools. Applications of pH measurement span across agriculture, medicine, and other industries, highlighting its importance in regulating bodily functions and maintaining safe water standards.