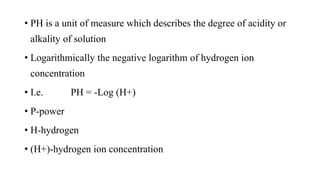
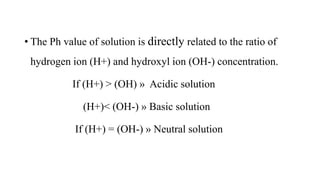
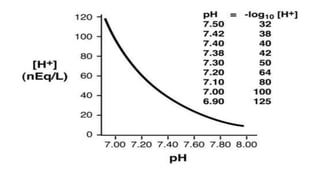
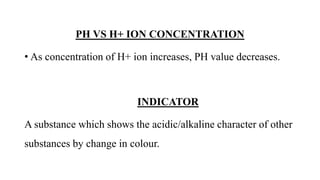
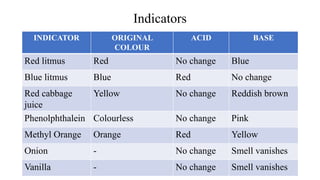
PH and buffers can be summarized in 3 sentences:
PH is a measure of acidity or alkalinity in solutions, determined by the concentration of hydrogen ions, with lower PH indicating more acidic solutions. Buffers resist changes in PH when acids or bases are added by maintaining an equilibrium between the acid/base forms. Common buffer systems used in biochemistry include acetate, borate, and bicarbonate buffers which are important for maintaining stable PH levels in processes like electrophoresis.




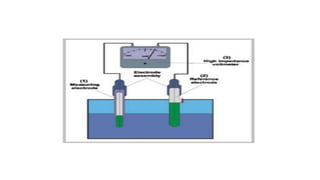
![PH METER
• It is the instrument that measures [H+] with concentration of
solution, using an ion selective electrode that ideally responds
to H+ ions
The 1st commercial pH meter was built in 1936.
pH meter – parts
A basic pH measuring system consists of
(1) the measuring electrode - a pH sensitive electrode,
(2) the reference electrode
(3) a high impedance voltmeter](https://image.slidesharecdn.com/phbuffersphmeter-230820094013-d2685322/85/PH-BUFFERS-PH-METER-pptx-10-320.jpg)

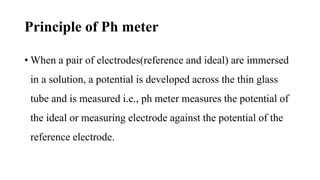
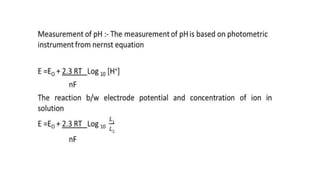
![Where
E → Measured potential
Eo → Functional potential
R → Molar gas constant [8.314 J mol-1k-1]
T → Absolute temperature [in K]
F → Faraday constant [96500 KJ/mole]
n → Ionic charge
L1 & L2 → concentration of solution 1 & 2](https://image.slidesharecdn.com/phbuffersphmeter-230820094013-d2685322/85/PH-BUFFERS-PH-METER-pptx-15-320.jpg)












