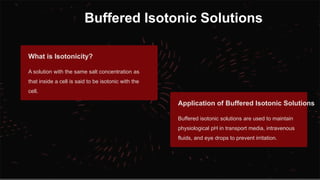
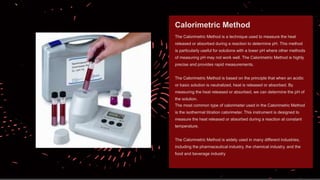
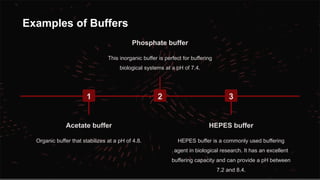
The document explains pH, buffers, and isotonic solutions, detailing how pH measures acidity or alkalinity with a scale from 0 to 14. It discusses methods to determine pH, such as the electrometric method using a glass electrode and the calorimetric method to measure heat changes. Furthermore, it highlights the importance of buffers in maintaining stable pH in various industries, including pharmaceutical and environmental science, and concludes with their applications in buffered isotonic solutions.





![Buffer Equation
The Henderson-Hasselbalch equation is commonly used to calculate the pH of a buffered solution:
pH = pKa + log([A-]/[HA])
Where "pKa" is the acid dissociation constant and "[A-]" and "[HA]" represent the concentrations
of the deprotonated and protonated forms of the buffer, respectively.](https://image.slidesharecdn.com/ph-buffers-and-isotonic-solutions-230628172335-599ba457/85/pH-Buffers-and-Isotonic-Solutions-pptx-8-320.jpg)
![Buffer Capacity
Buffer capacity is the amount of acid or base that can be added/removed without any significant impact on the pH of the solution. It tells us how strong the
buffer is. Buffers with higher capacity are better suited to stabilize pH changes in complex systems.
The equation for buffer capacity is:
β = Δ[base]/ΔpH = Δ[acid]/ΔpH
Alka-Seltzer
The buffer in Alka-Seltzer neutralizes excess gastric acid in the stomach.
Blood Buffering Systems
The bicarbonate buffer system in blood maintains its pH in the narrow range
of 7.35 to 7.45.](https://image.slidesharecdn.com/ph-buffers-and-isotonic-solutions-230628172335-599ba457/85/pH-Buffers-and-Isotonic-Solutions-pptx-9-320.jpg)