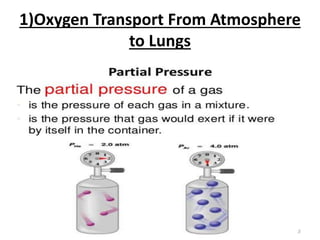
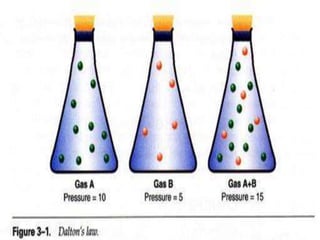
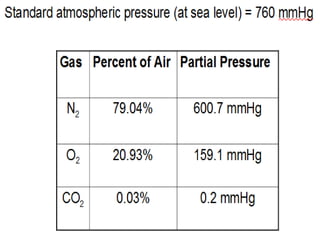
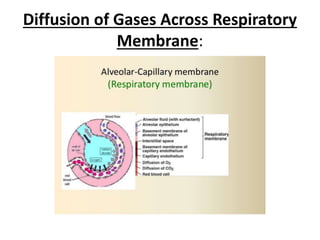
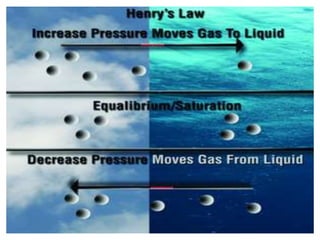
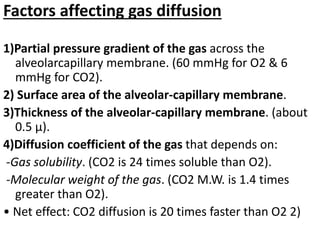
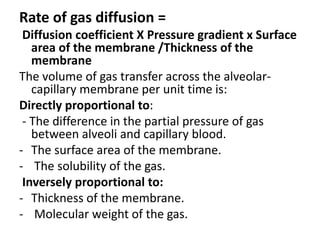
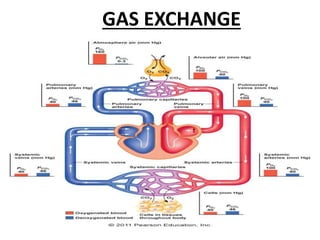
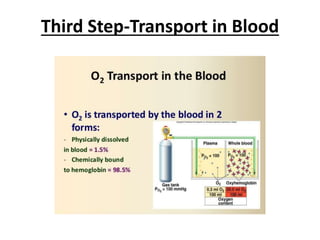
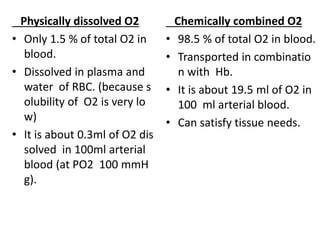
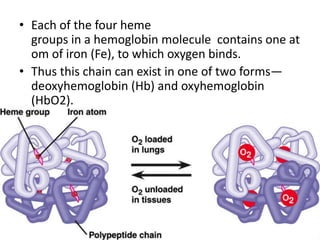
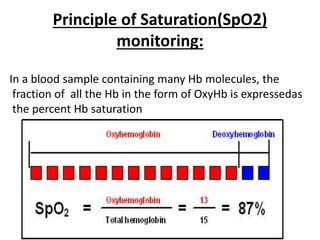
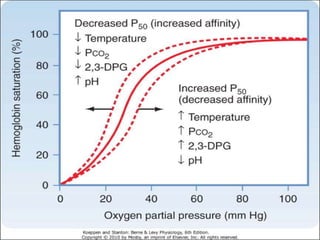
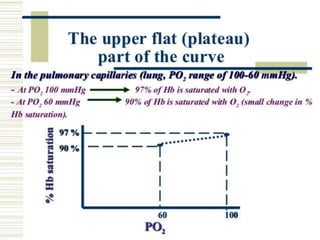
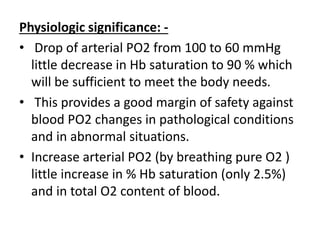
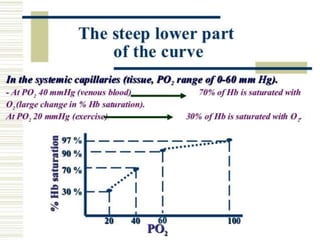
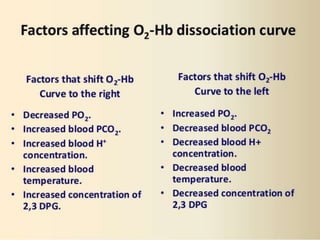
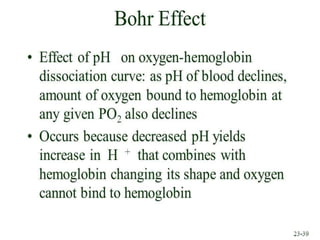
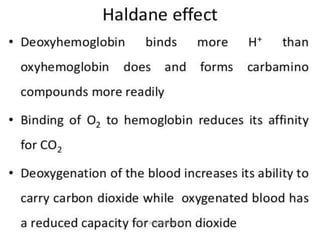
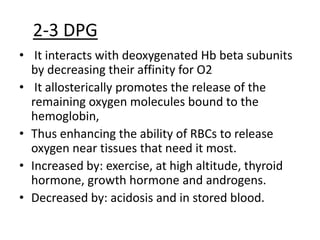
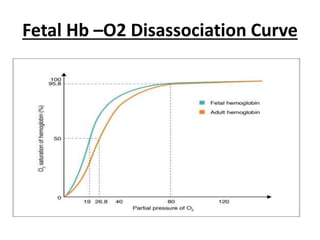
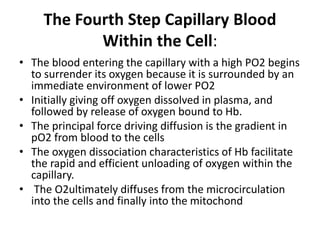
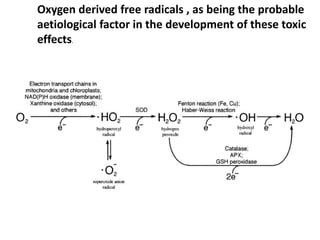
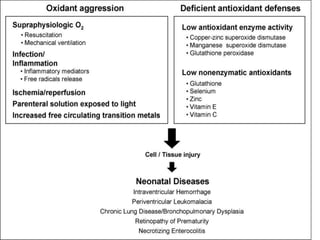
This document discusses oxygen transport from the atmosphere to tissues. It describes how oxygen is absorbed in the lungs and bound to hemoglobin to be carried by the blood. The oxyhemoglobin dissociation curve is explained, showing how hemoglobin releases oxygen in tissues. Factors affecting oxygen diffusion and binding such as partial pressure gradients, carbon dioxide levels, 2,3-DPG, and fetal hemoglobin are covered. The document also briefly discusses oxygen toxicity and ischemia-reperfusion injury.