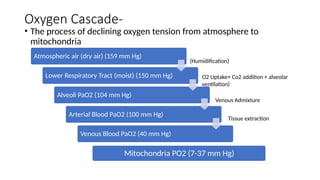
The document explains the oxygen dissociation curve, which illustrates how oxygen binds to hemoglobin (Hb) in blood, primarily for transport. It details the significance of partial pressure in oxygen transport, the dynamics of oxygen binding, and the effects of various physiological conditions on Hb's affinity for oxygen. Additionally, it addresses concepts such as the Haldane and Bohr effects that describe oxygen uptake and release in the lungs and tissues, respectively.