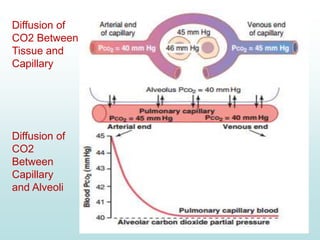
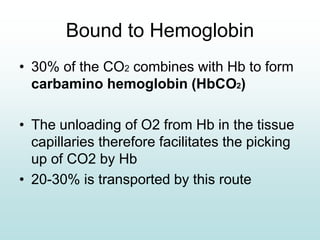
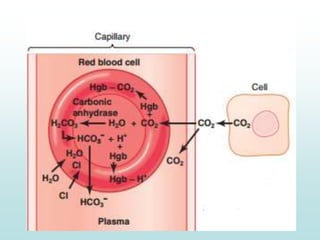
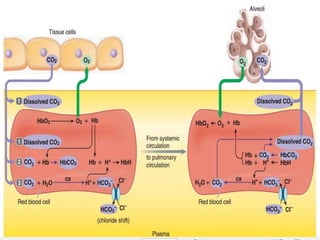
Carbon dioxide is transported in the blood primarily in three ways: physically dissolved (7-10%), bound to hemoglobin (20-30%), and as bicarbonate ions (60-70%). The conversion of CO2 to bicarbonate occurs in red blood cells with the help of carbonic anhydrase, facilitated by a chloride shift mechanism. The Haldane effect describes how oxygen binding to hemoglobin displaces CO2 from the blood, enhancing its release into the alveoli.