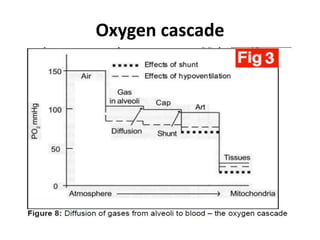
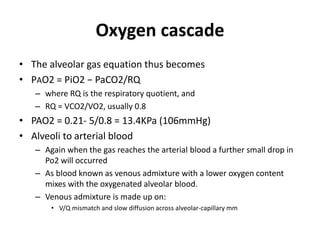
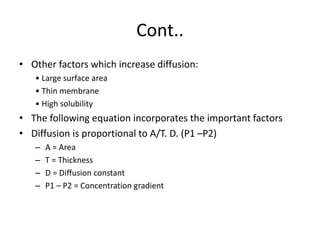
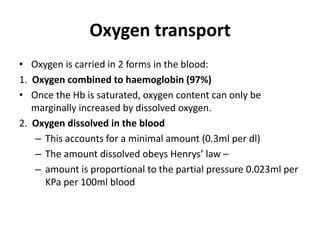
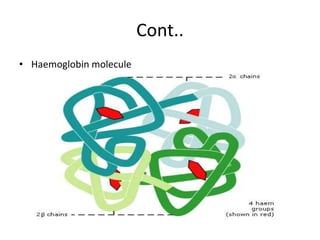
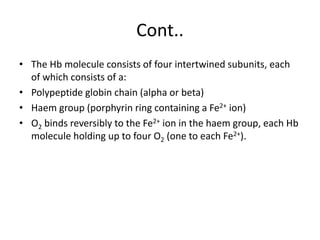
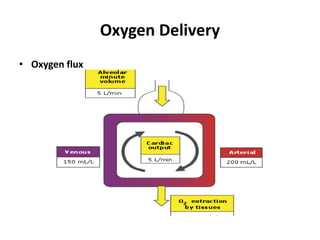
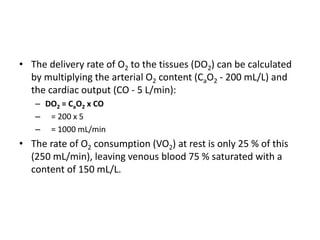
This document discusses oxygen transport and consumption in the human body. It begins by outlining the learning objectives, which are to calculate oxygen consumption at rest, explain how oxygen is carried in the blood and measured, recognize factors that increase oxygen consumption, and identify how more oxygen can be delivered to tissues when needed. It then provides details on oxygen transport from the air to mitochondria via different mechanisms, how oxygen is bound to hemoglobin and affects its binding curve, and how the body can increase oxygen delivery through various physiological responses.
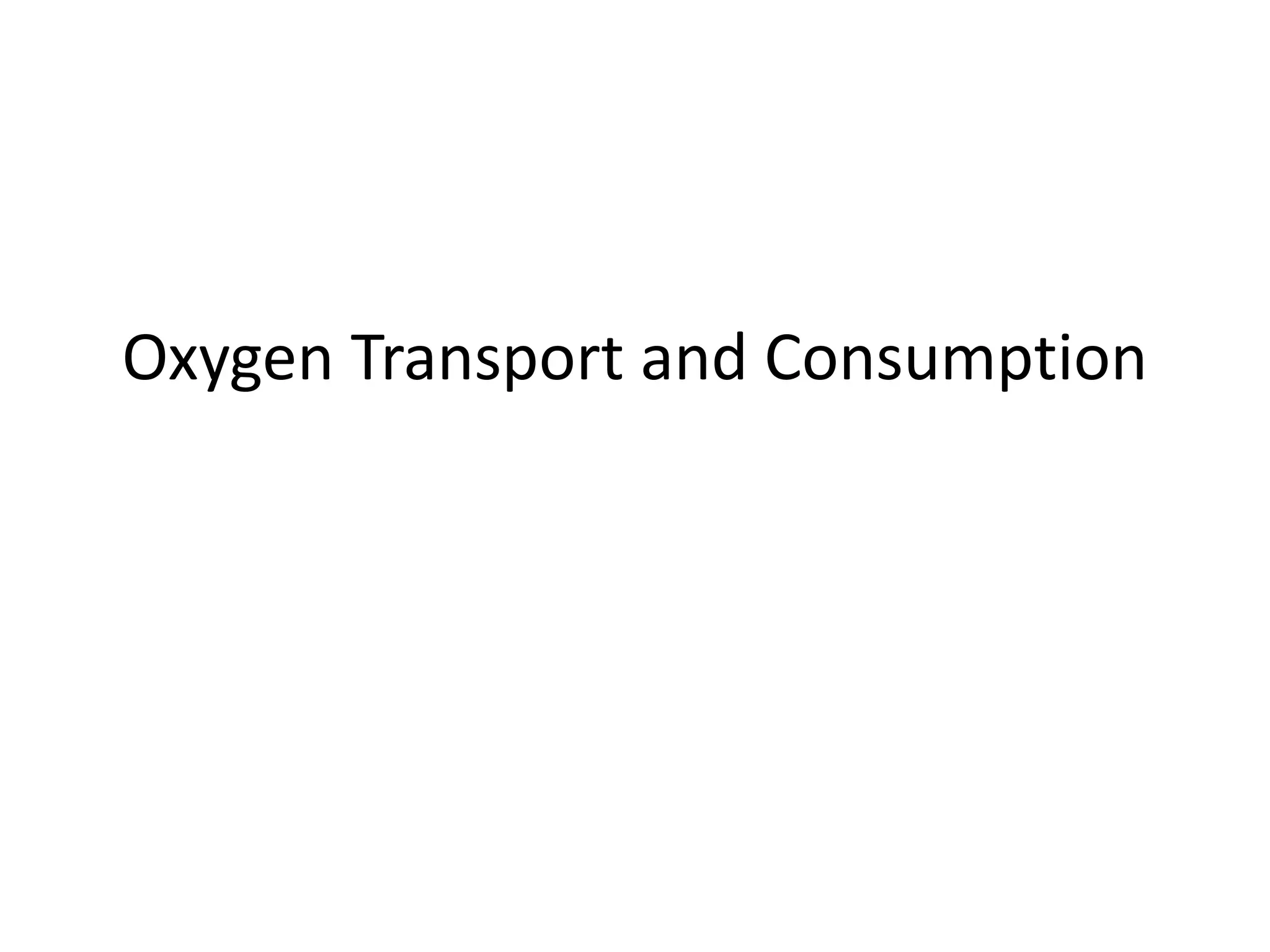









![O2 content
• O2 content is the truest measure of oxygen present but is
difficult to measure directly and so is normally estimated from
the other values.
Calculating O2 content
• When fully saturated, 1 g of Hb holds 1.34 mL O2.
• Assuming a Hb concentration of 150 g/L (15 g/dL), we can
calculate the oxygen content in arterial blood (CaO2).
CaO2= Hb-bound + dissolved
= ([Hb] x 1.34 x satn) + (0.23 x PO2)
= (150 x 1.34 x 0.98) + (0.23 x 13)
= 197 + 3
= 200 mL/L](https://image.slidesharecdn.com/oxygentransport-190215174401/85/Oxygen-transport-27-320.jpg)

![Cyanosis
• It is detected clinically when ≥ 5 g/dL deoxy-Hb can be seen in
the skin or mucous membranes.
• With a [Hb] of 15 g/dL this is 33 % of the total, with the
remaining 67 % being saturated.
• In practice, patients appear cyanosed when the pulse
oximetry reading is much higher, around 85 %.
• Cyanosis is seen in capillary blood, whilst a pulse oximeter
reading is based on the arterial value, which will be
substantially higher because:
– PO2 is slightly higher in the artery than the capillary
– The saturation curve in the capillary has a small right shift (Bohr effect)](https://image.slidesharecdn.com/oxygentransport-190215174401/85/Oxygen-transport-29-320.jpg)