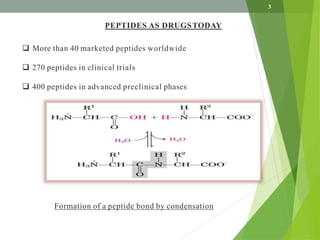
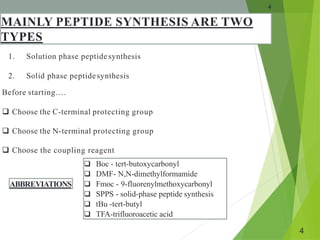
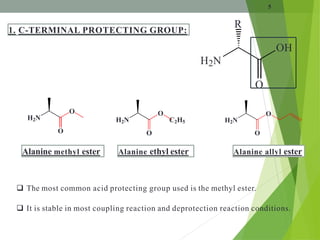
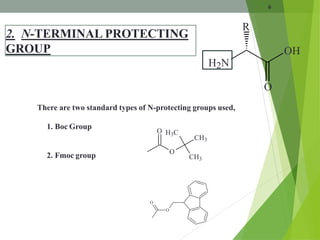
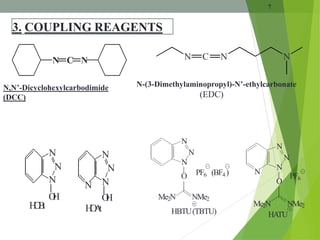
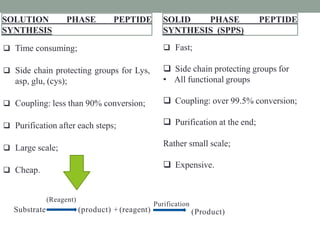
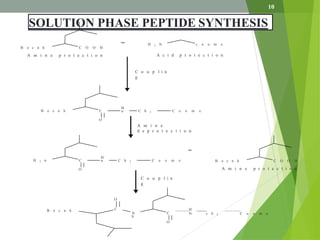
The document presents a comprehensive overview of segment and sequential strategies for solution phase peptide synthesis. It discusses protecting groups, the differences between solid and solution phase synthesis, various coupling reagents, and the advantages of linear versus convergent strategies. The case study section emphasizes automated and manual synthesis methods, along with applications and analytical techniques used in peptide synthesis.