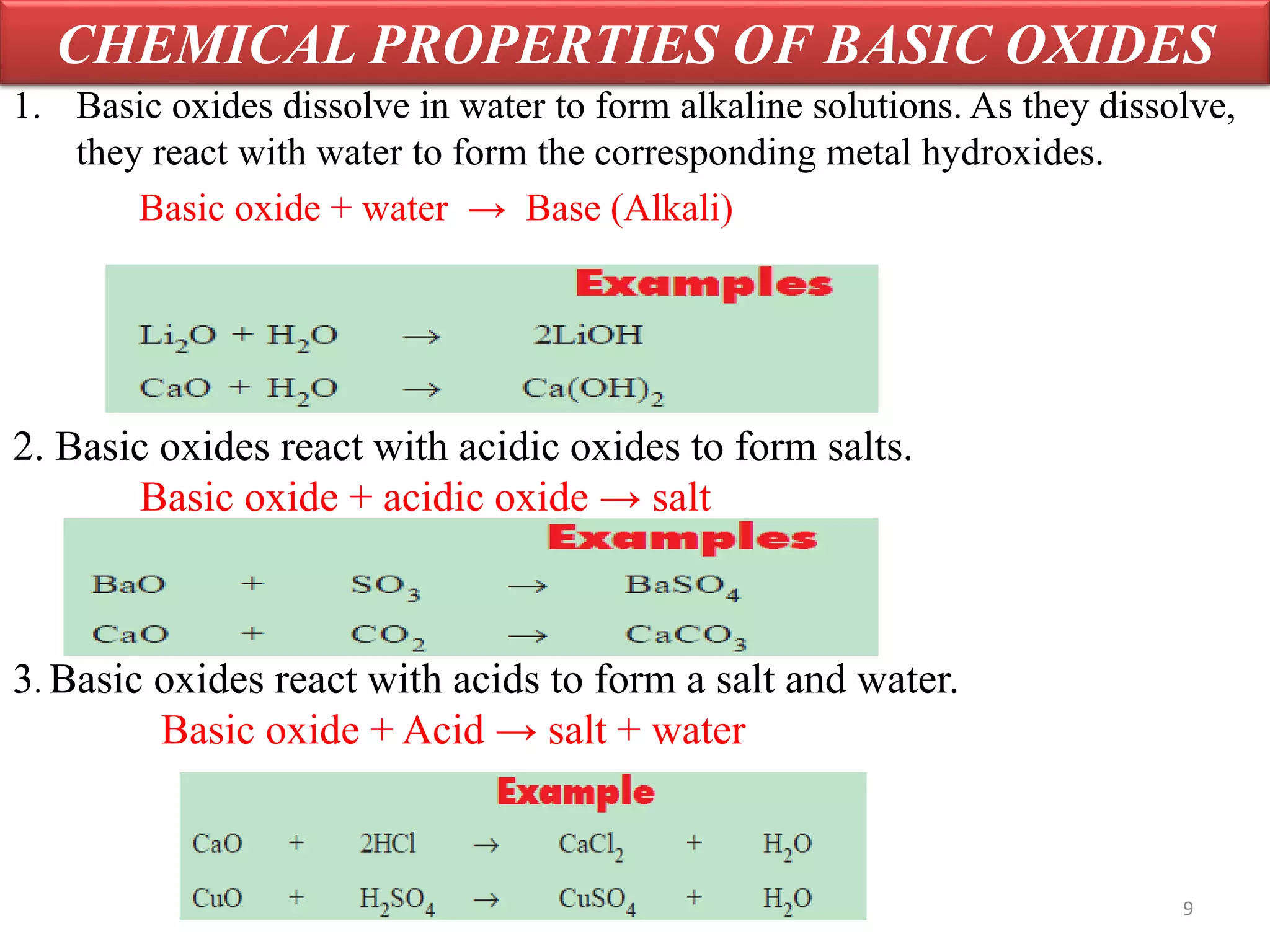
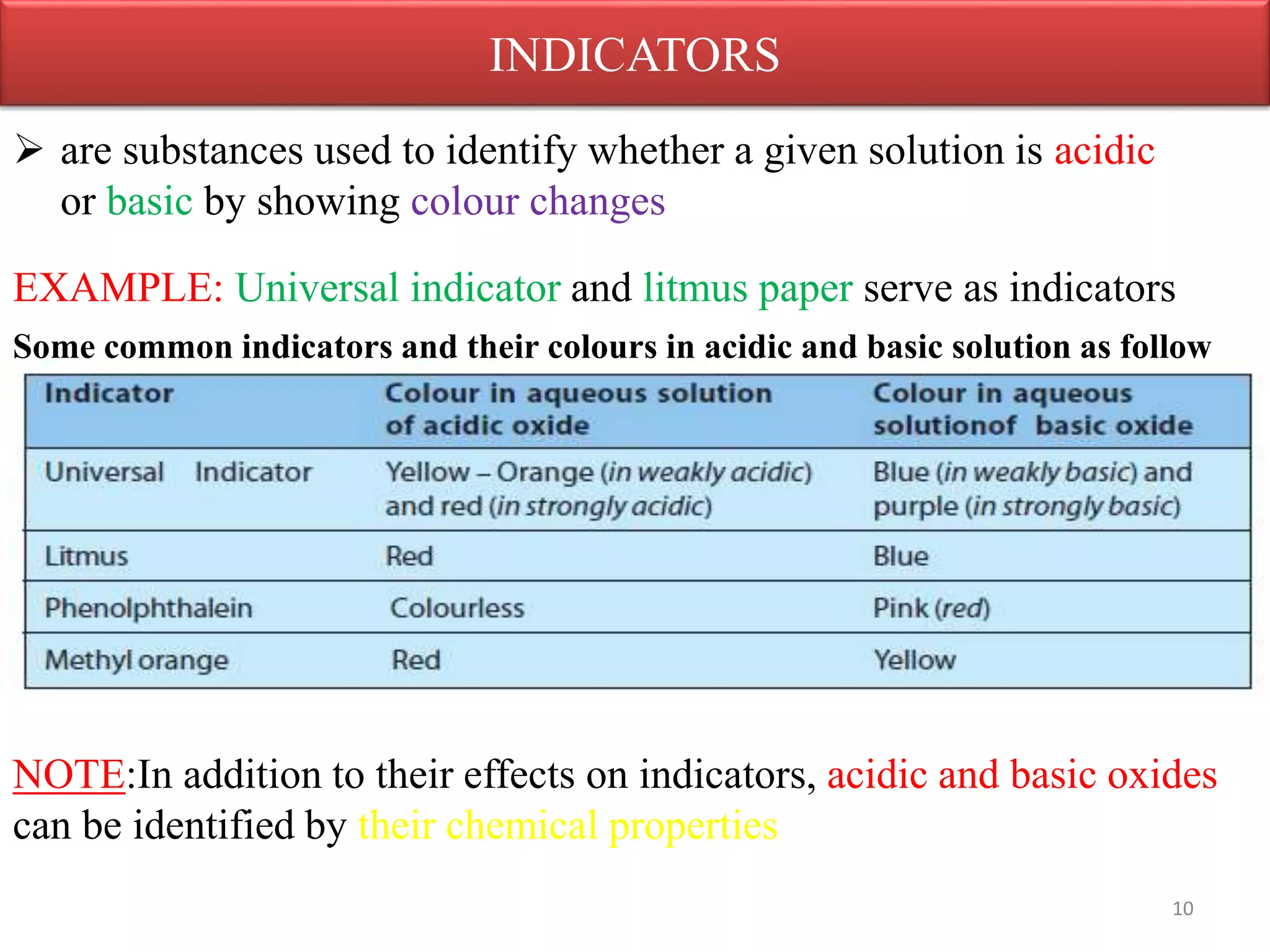
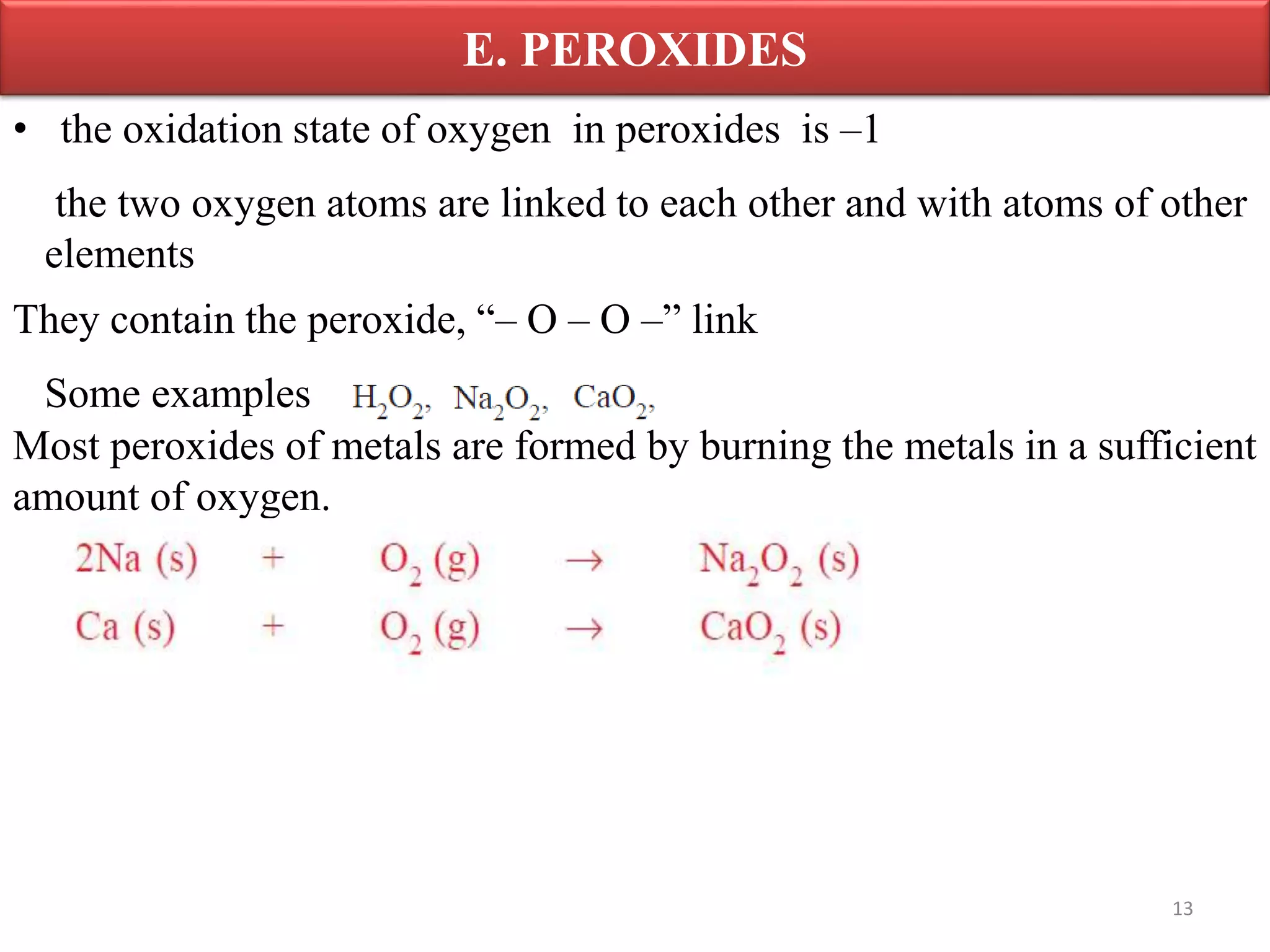
Inorganic compounds are compounds found in non-living things and consist mostly of mineral constituents of the earth. They can be classified based on the elements they contain, such as metals or non-metals, or the group they belong to like sulfates or nitrates. Important classes of inorganic compounds discussed include oxides, which contain oxygen and another element and can be acidic, basic, amphoteric, neutral or peroxides depending on their chemical properties.