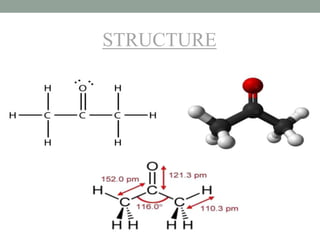
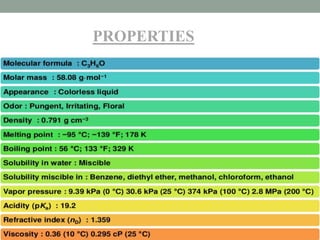
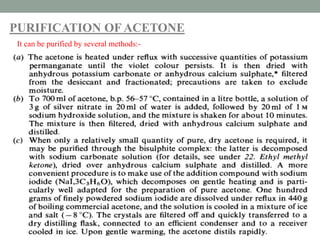
Shubham Sharma presented on acetone. Acetone, also known as propan-2-one, is the simplest ketone with the formula (CH3)2CO. It serves as an important industrial and laboratory solvent that can dissolve many compounds. The presentation covered the structure, properties, material safety data sheet, and common impurities of acetone, as well as several purification methods.