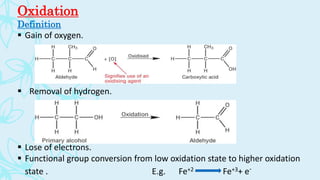
This document discusses oxidation reactions with osmium tetroxide (OsO4). It defines oxidation as the gain of oxygen, removal of hydrogen, or loss of electrons. OsO4 is described as a highly toxic volatile oxidizing agent with an oxidation state of +8 that is used to introduce hydroxyl groups into organic compounds through cis-hydroxylation of carbon-carbon double bonds. The mechanism involves the pi electrons of the alkene forming a 5-membered cyclic osmate ester intermediate. Hydroxide then liberates the cis-diol product while reducing the oxidation state of osmium from +8 to +6.