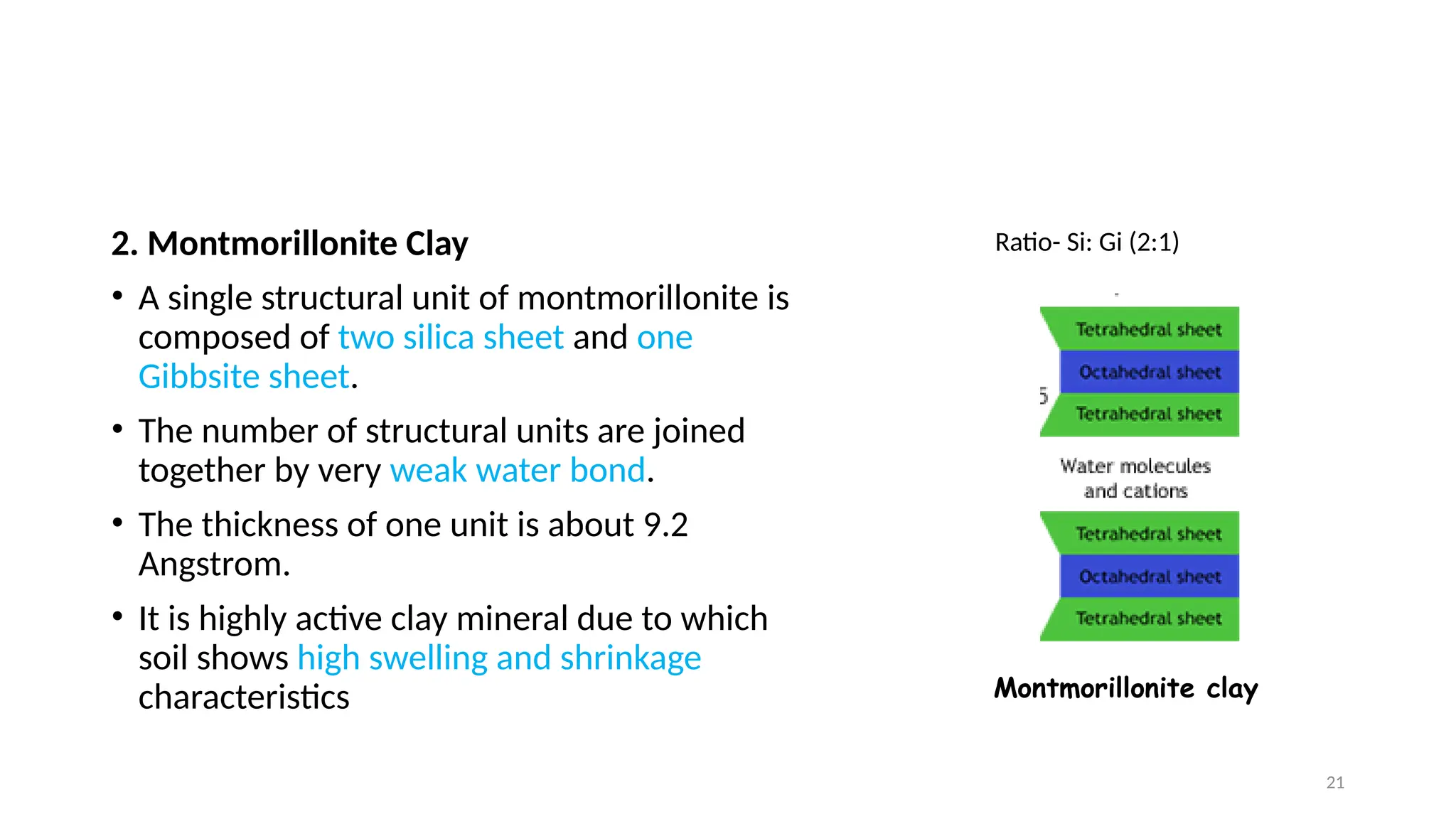
The document discusses clay chemistry, focusing on its types, properties, and applications in drilling fluids. It outlines the characteristics of colloidal systems, the significance of clay mineralogy, various clay mineral types (including kaolinite, montmorillonite, and illite), and the mechanisms of ion exchange and swelling. Additionally, it examines the effects of electrostatic double layers and flocculation in the context of clay interactions in aqueous environments.




























![30
Origin and occurrence of clay minerals
• Clay minerals originate from the degradation of igneous rocks in situ.
• The parent minerals are the micas, the feldspars, [(CaO) (K20)Al2036Si02]; and
ferromagnesium minerals, such as horneblende [(Ca, Na2
)2 (Mg, Fe, Al)s (Al, Si)8022
(OH,F)2]
• Bentonite is formed by the weathering of volcanic ash.
• Bentonite was originally defined as a clay produced by in situ alteration of volcanic ash
to montmorillonite
• The main factors are climate, topography, vegetation, and time of exposure.](https://image.slidesharecdn.com/unit3claychemistry-240827161013-2ca2eead/75/Unit-3-Clay-Chemistry-for-drilling-fluids-pptx-30-2048.jpg)


![33
Ion Exchange
• Where [A]s and [B]s are the molecular concentration of the two species of ions in
the solution, and [A]c and [B]c are those on the clay.
• K is the ion exchange equilibrium constant
• Example: when K is greater than unity, A is preferentially adsorbed.](https://image.slidesharecdn.com/unit3claychemistry-240827161013-2ca2eead/75/Unit-3-Clay-Chemistry-for-drilling-fluids-pptx-33-2048.jpg)














































