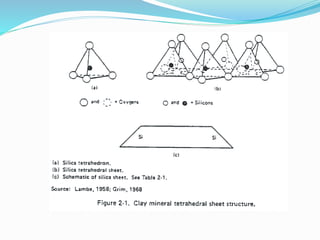
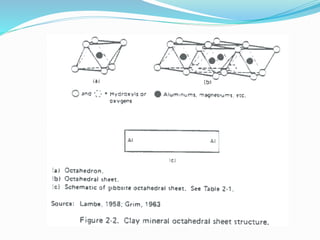
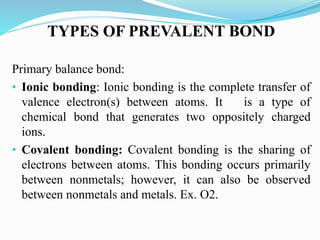
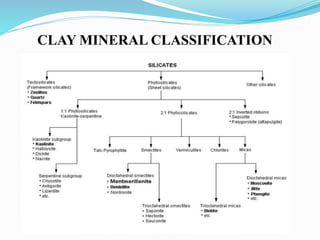
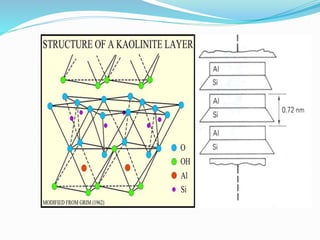
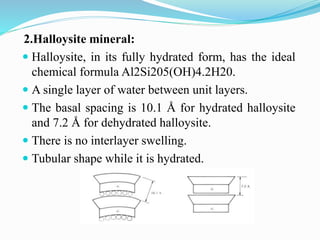
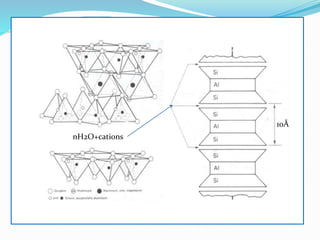
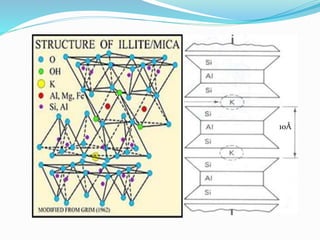
This document presents information on clay minerals. It begins with an introduction stating that clay minerals are formed through chemical weathering and are found in shales. It then discusses the basic structural units of clay minerals, which are tetrahedral and octahedral sheets. The document outlines different types of bonding in clay minerals and provides a classification of clay minerals including kaolinite, halloysite, montmorillonite and illite. In conclusion, it restates that clay minerals are layer silicates formed by weathering and describes their layered atomic structure and different bonding types.