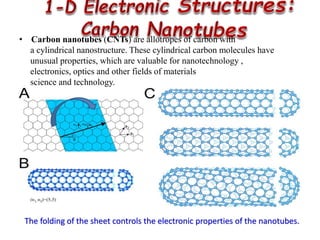
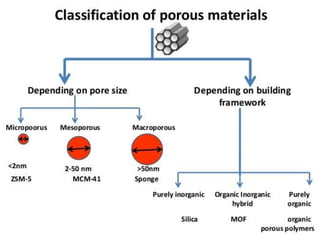
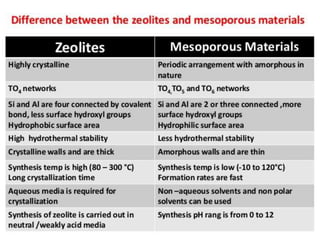
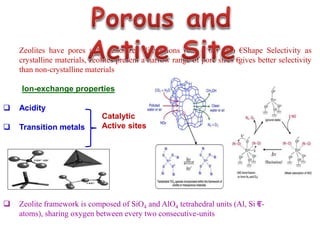
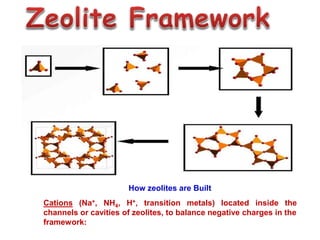
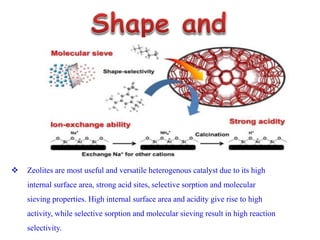
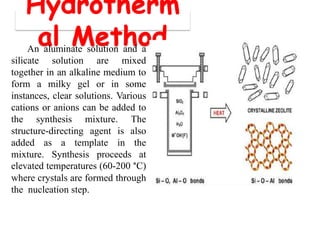
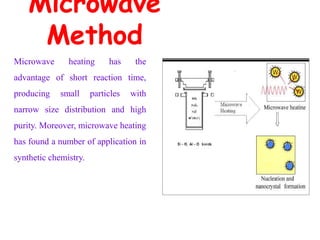
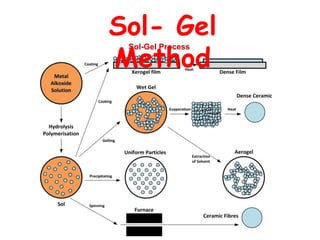
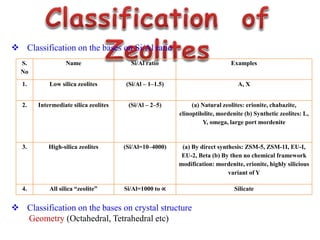
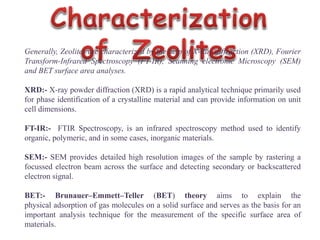
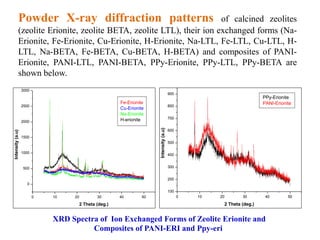
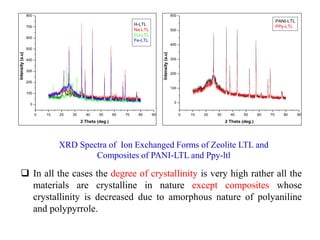
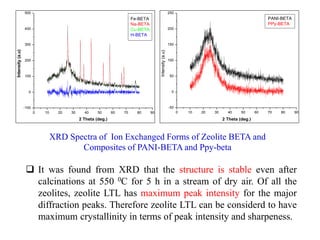
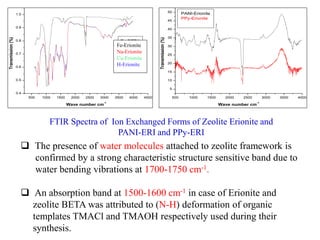
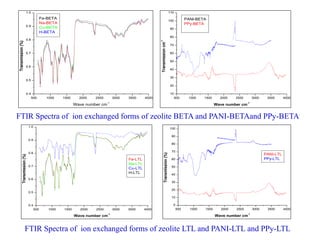
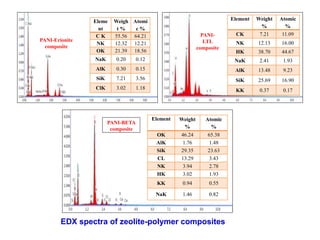
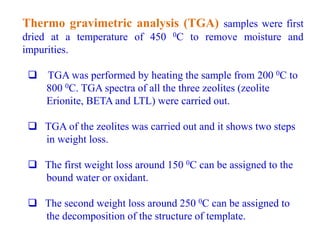
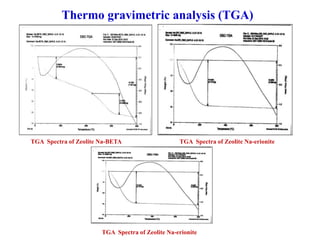
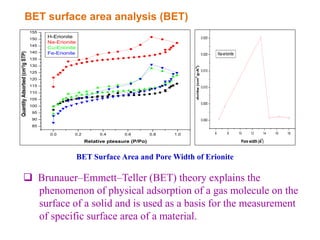
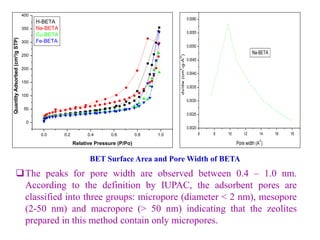
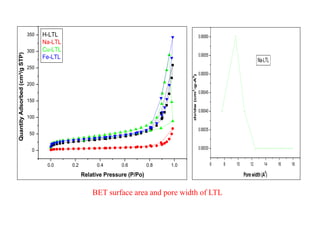
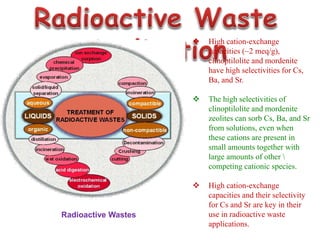
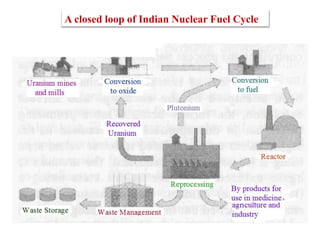
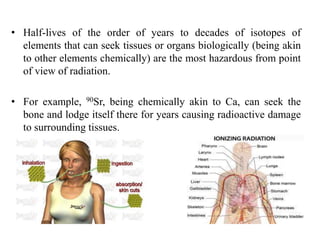
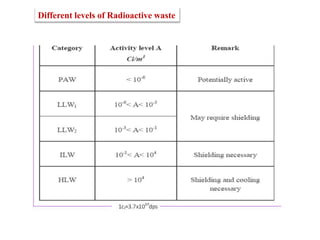
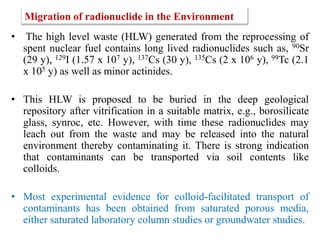
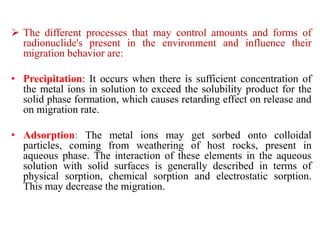
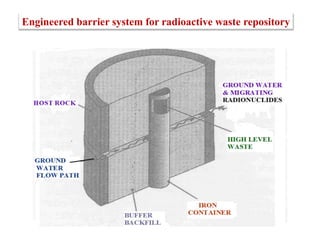
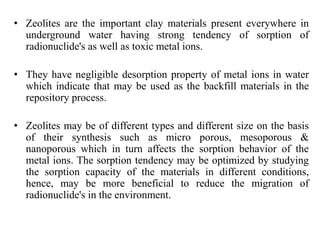
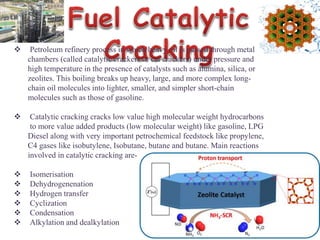
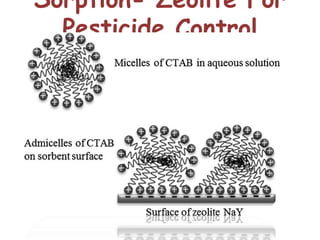
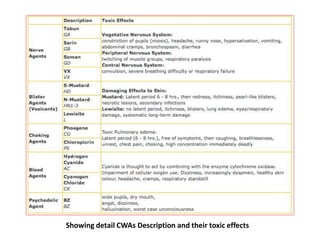
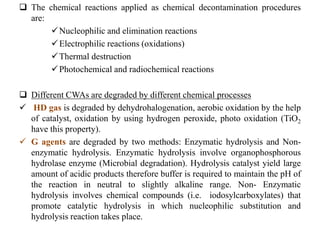
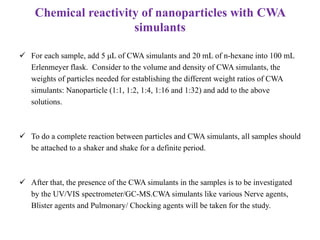
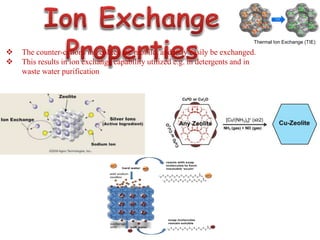
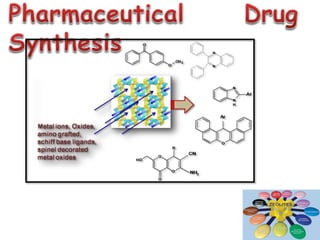
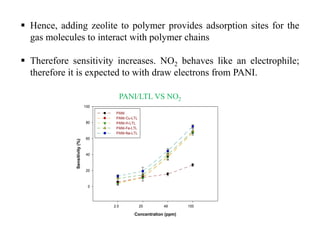
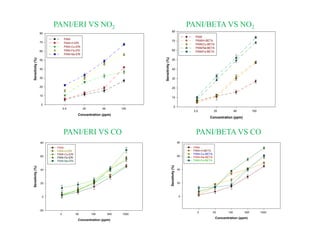
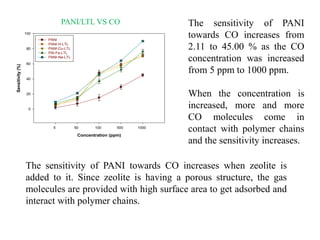
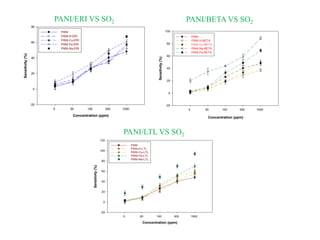
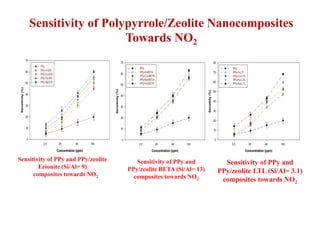
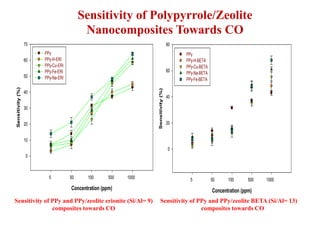
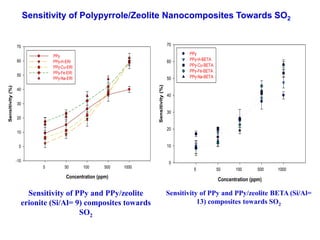
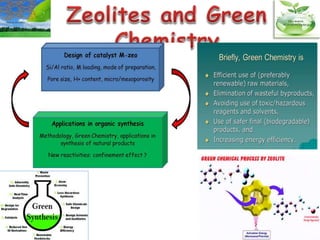
This document discusses different types of materials, including metals, polymers, ceramics, composites, and smart materials. It provides details on their key properties and examples. Metals are good conductors of heat and electricity, while polymers are made of long molecular chains that can be cross-linked. Ceramics are inorganic materials made by heating materials like silica and clay. Composites have improved properties from combining materials with a matrix and reinforce. Smart materials change properties in response to stimuli like stress, temperature, or electric fields.