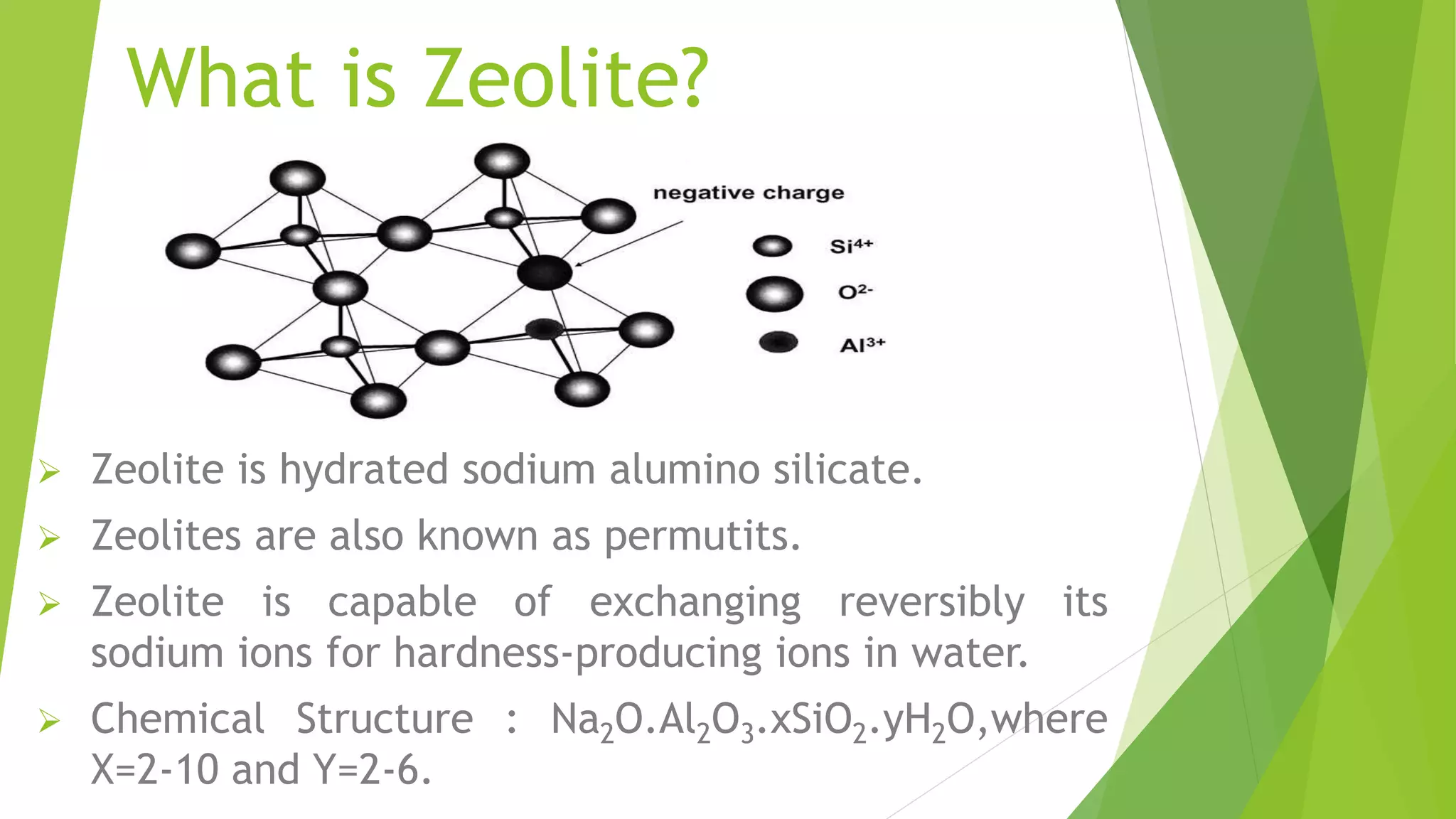
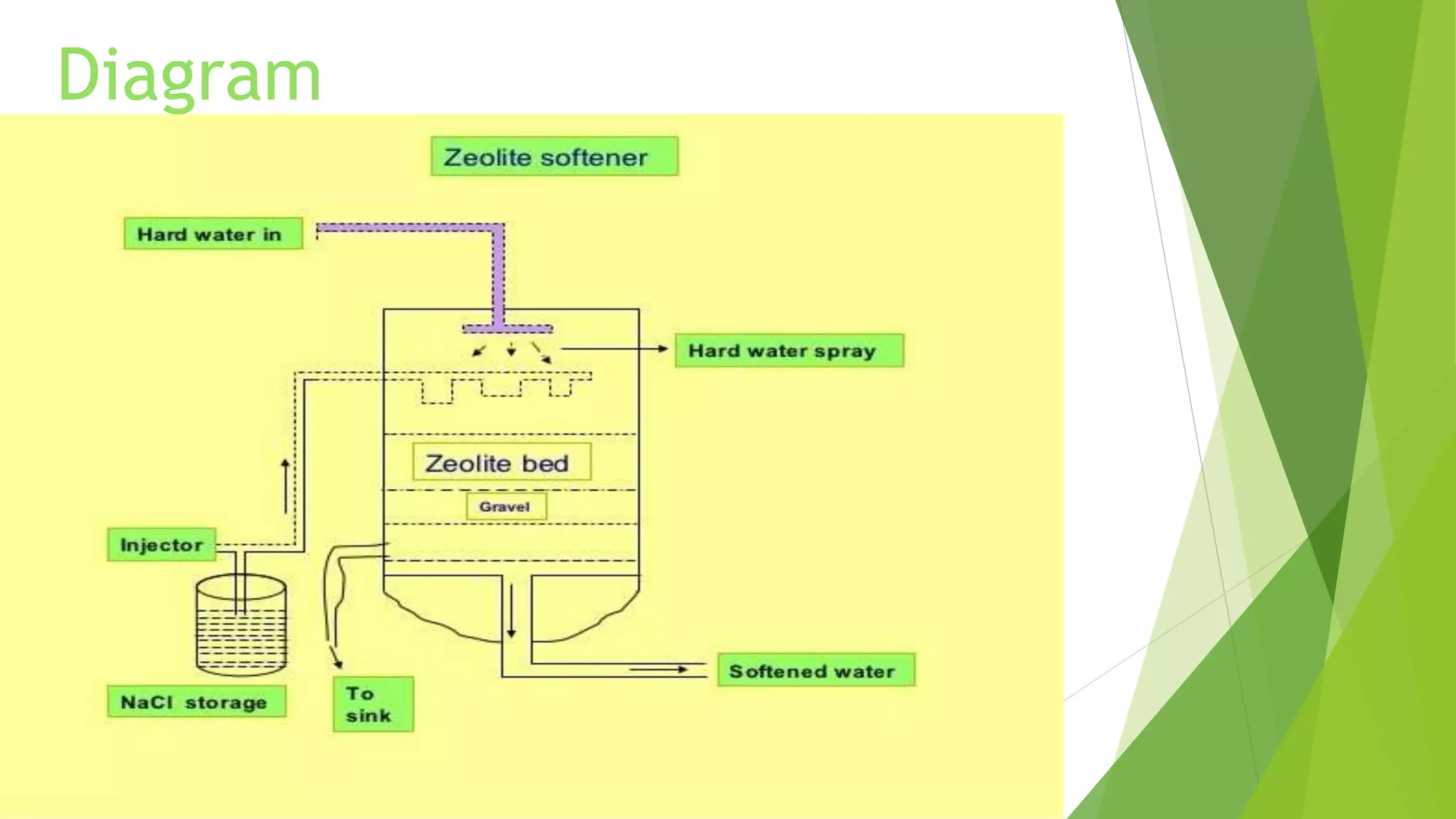
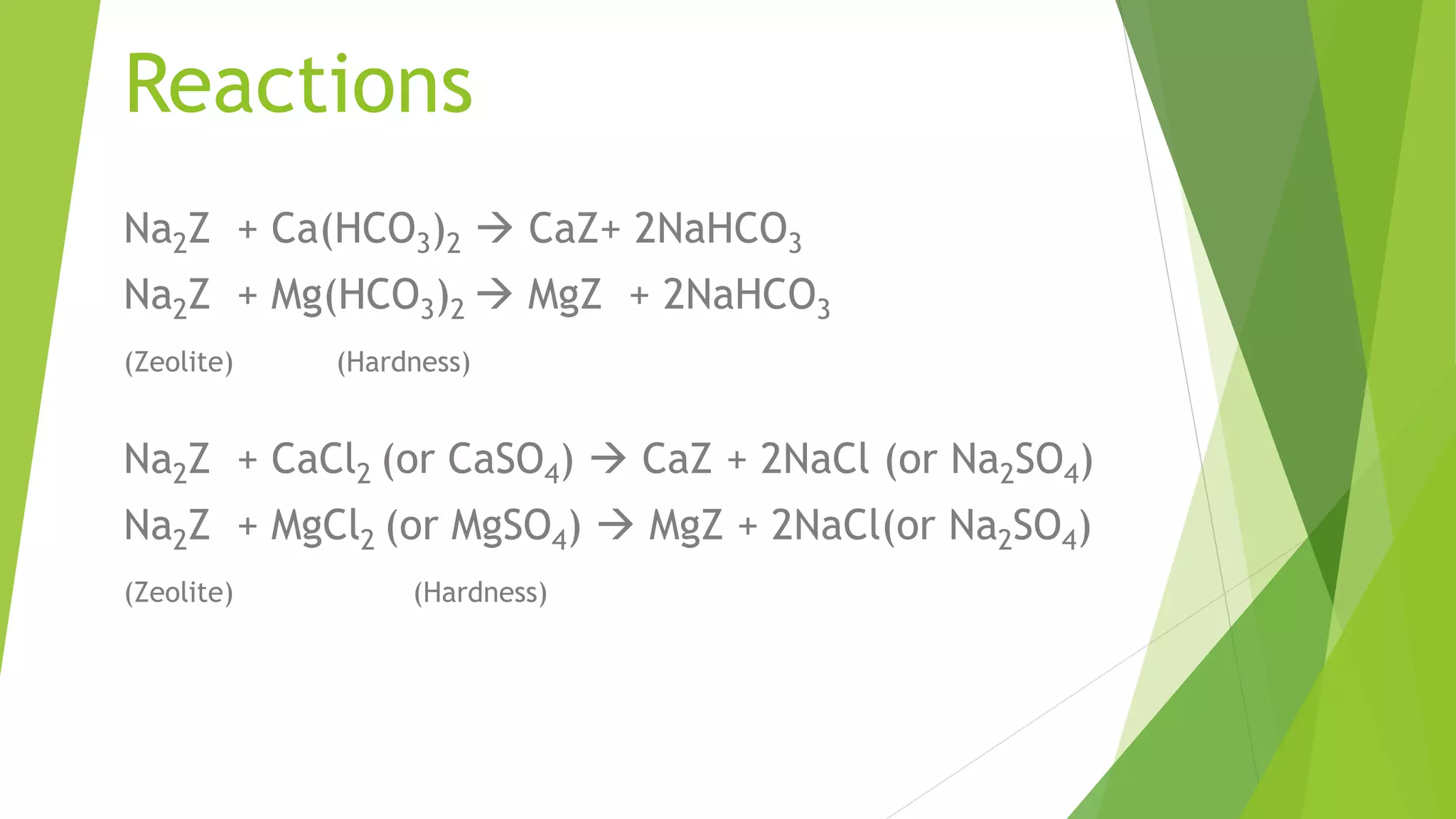
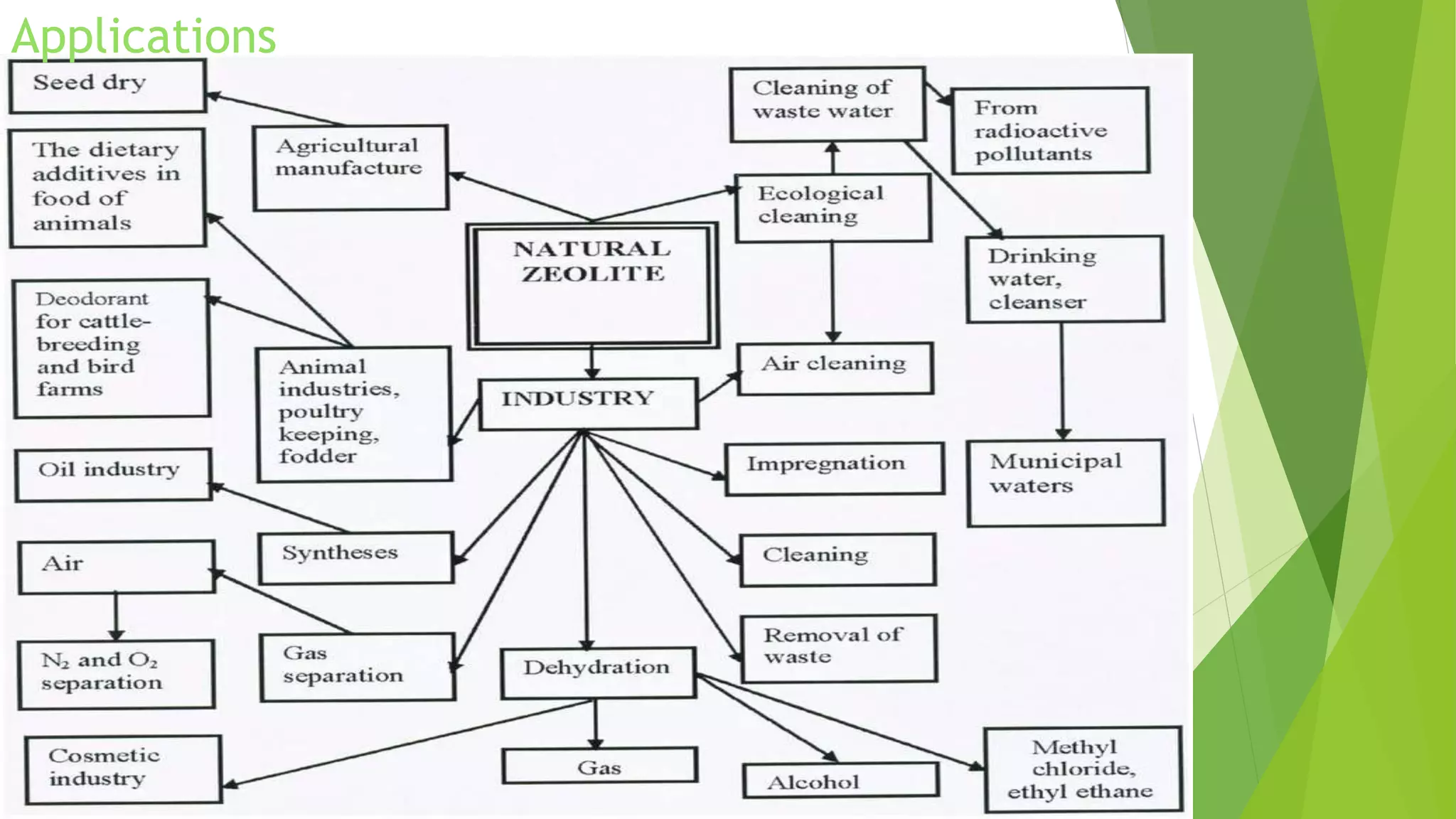
Zeolite is a hydrated sodium alumino silicate used for water softening by exchanging sodium ions for hardness ions like calcium and magnesium. It comes in two forms: natural (non-porous) and synthetic (porous), and is used in various applications including aquaculture, catalysis, and gas separation. The process effectively reduces water hardness but has limitations, such as sensitivity to turbidity and mineral acids, while offering advantages like a compact system and minimal impurity formation.








![References
[1] Caputo D, Pepe F (2007) Experiments and data processing of ion exchange equilibria involving
Italian natural zeolites: a review. Micropor.Mesopor.Mater. 105:222-231. Natural Zeolites in Water
Treatment – How Effective is Their Use 107
[2] Misaelides P (2011) Application of natural zeolites in environmental remediation: A short
review. Micropor.Mesopor.Mater. 144:15-18.
[3] Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review.
J.Environ.Manage.92:407-418.
[4] Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater
treatment. Chem. Engin.J. 156:11-24.
[5] Moussavia G, Talebi S, Farrokhi M, Sabouti RM (2011) The investigation of mechanism, kinetic
and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Engin.J.
171:1159-1169.
[6] Inglezakis VJ, Doula MK, Aggelatou V, Zorpas AA (2010) Removal of iron and manganese from
underground water by use of natural minerals in batch mode treatment. Desalin. Water Treat.
18:341-346.
[7] Doula MK (2009) Simultaneous removal of Cu, Mn and Zn from drinking water with the use of
clinoptilolite and its Fe-modified form. Water Res. 43:15:3659-3672](https://image.slidesharecdn.com/basicofzeoliteapplications-210811080016/75/Basic-of-zeolite-its-applications-14-2048.jpg)
