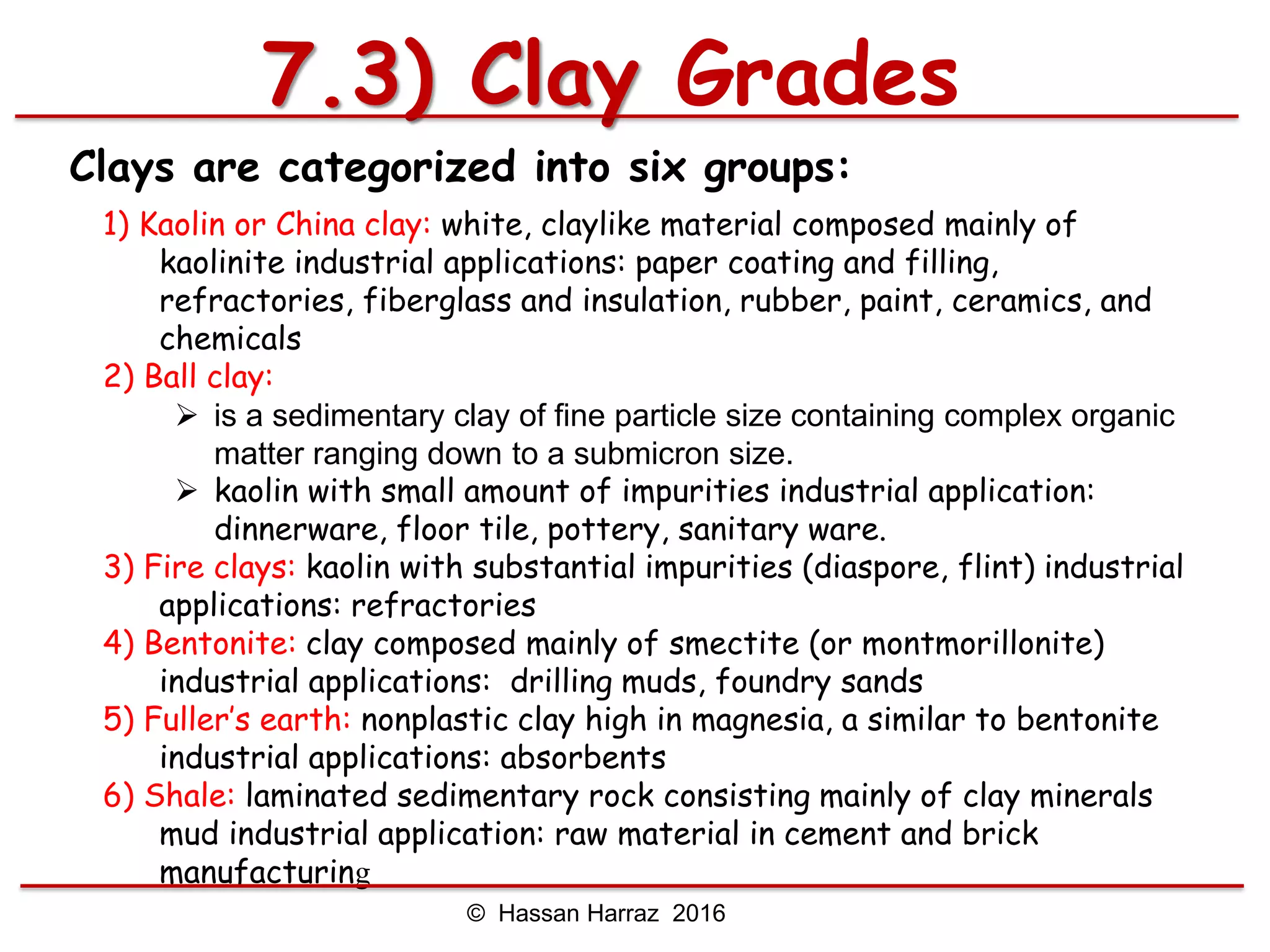
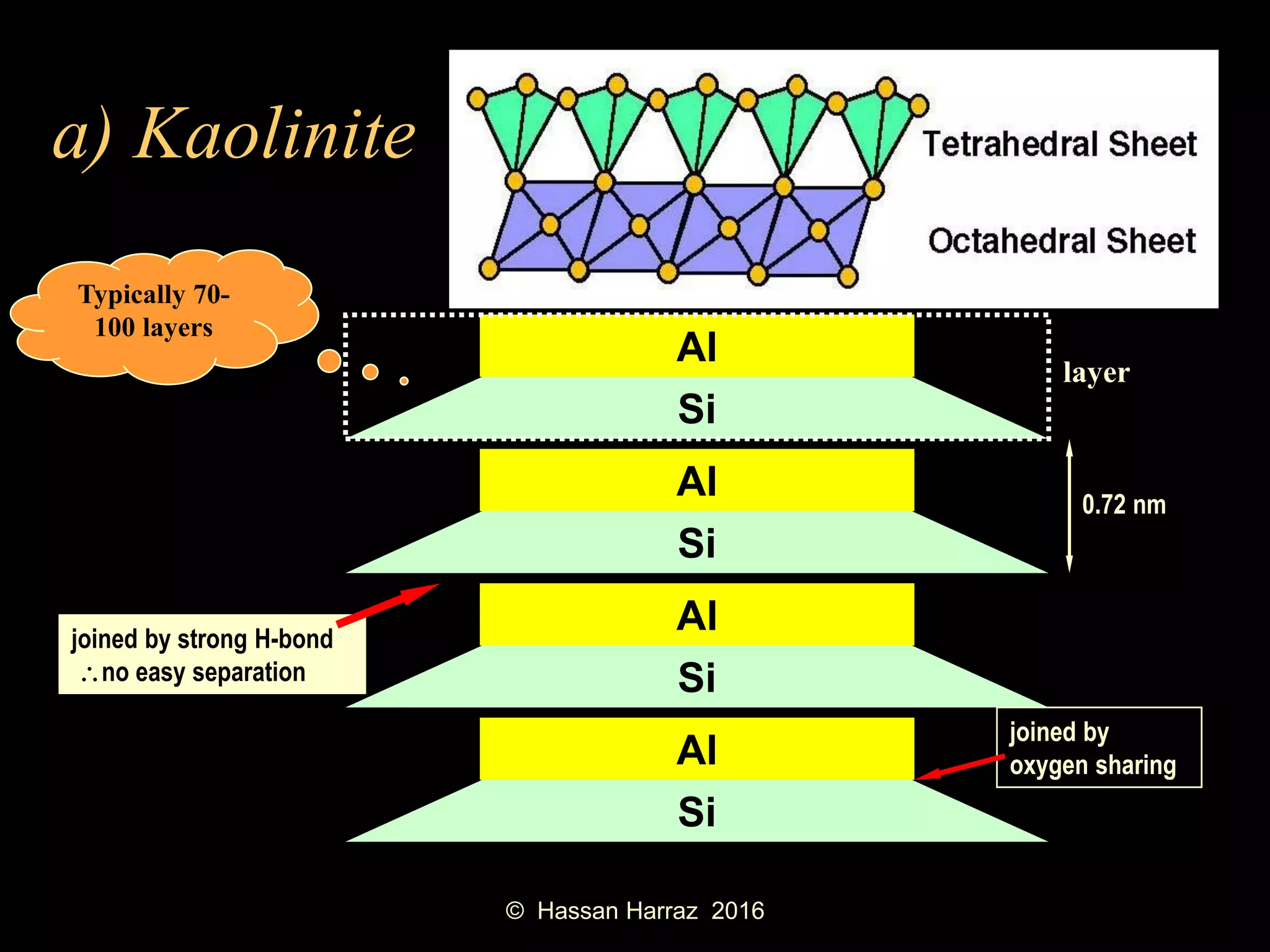
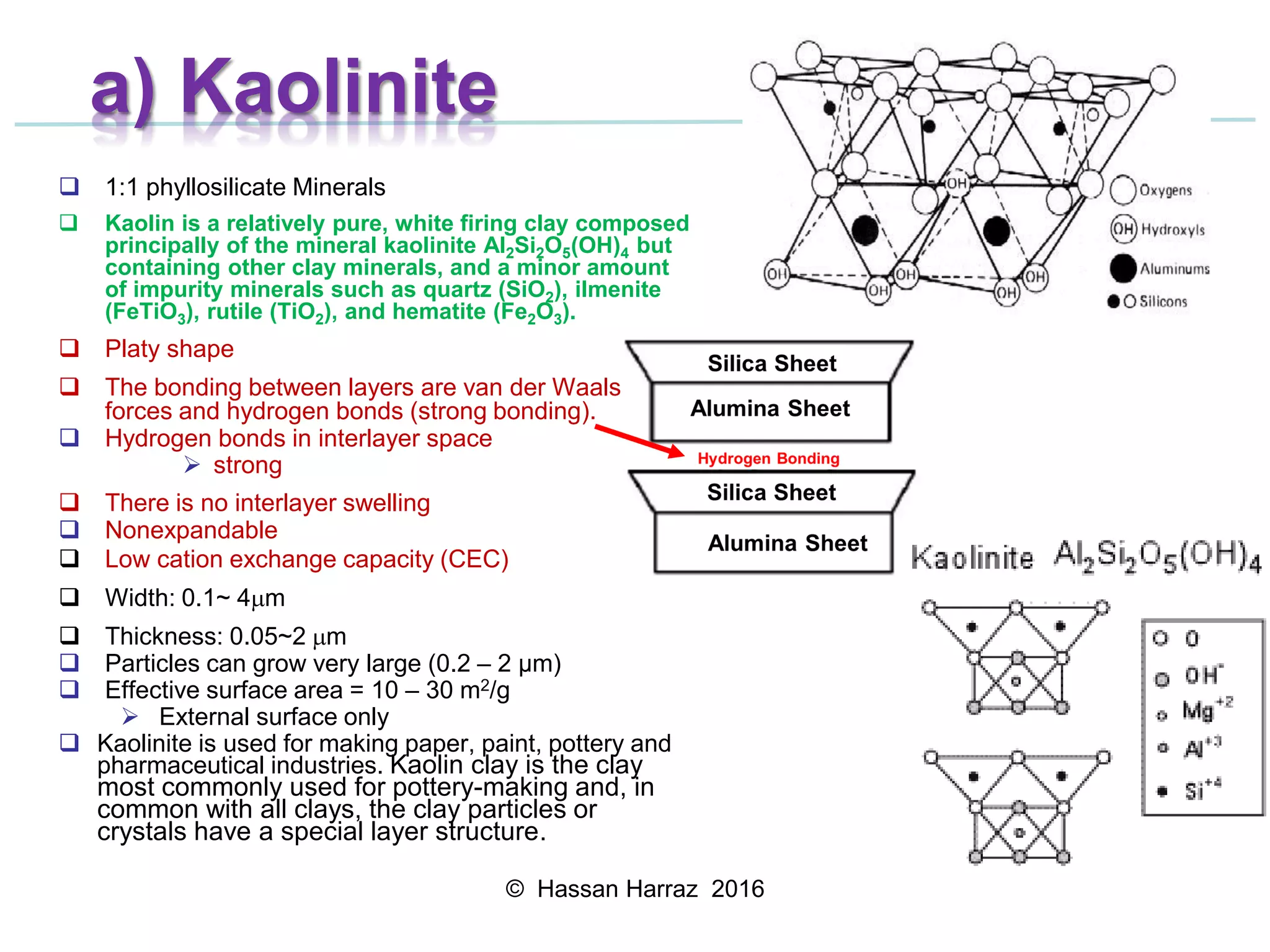
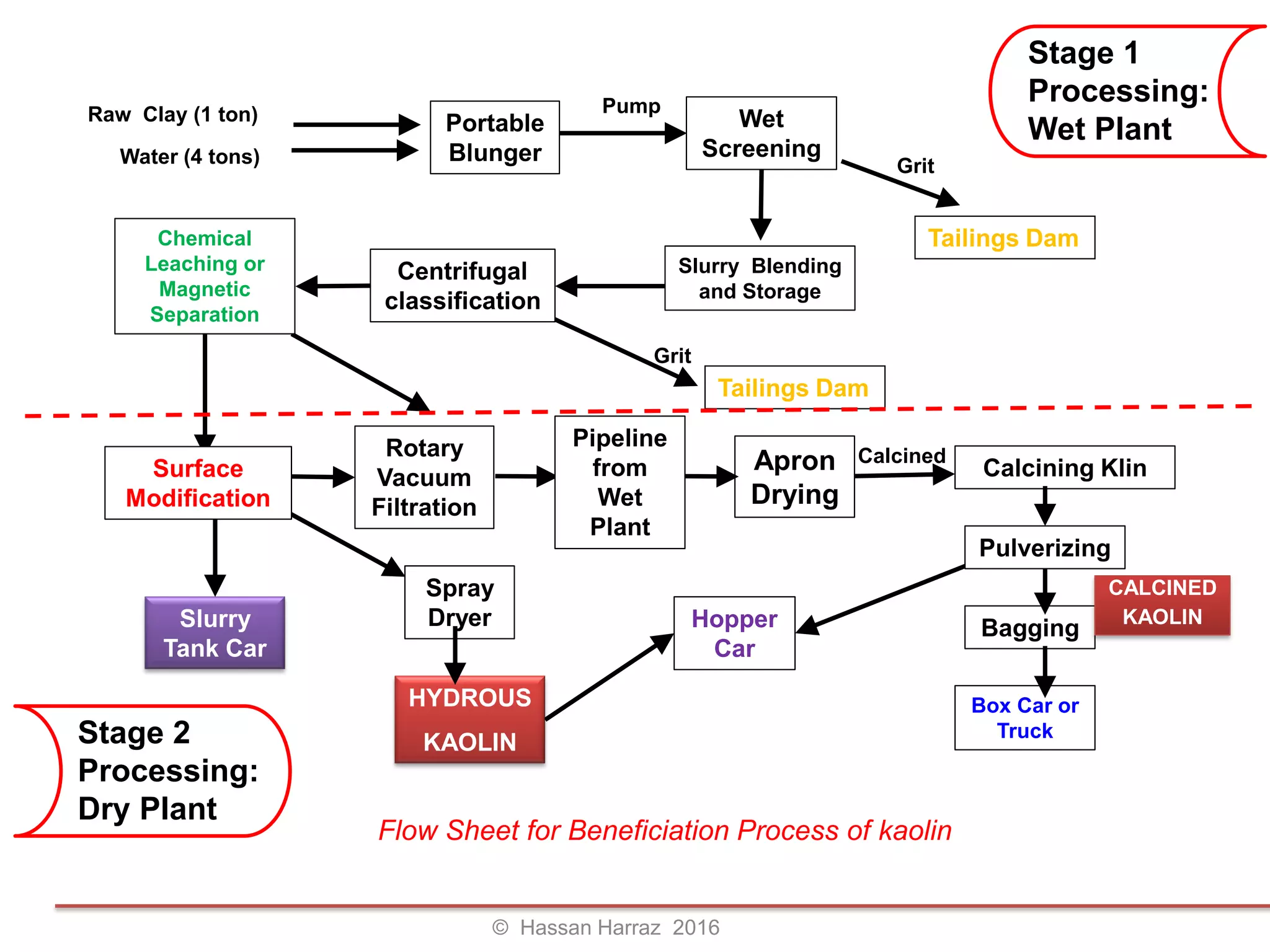
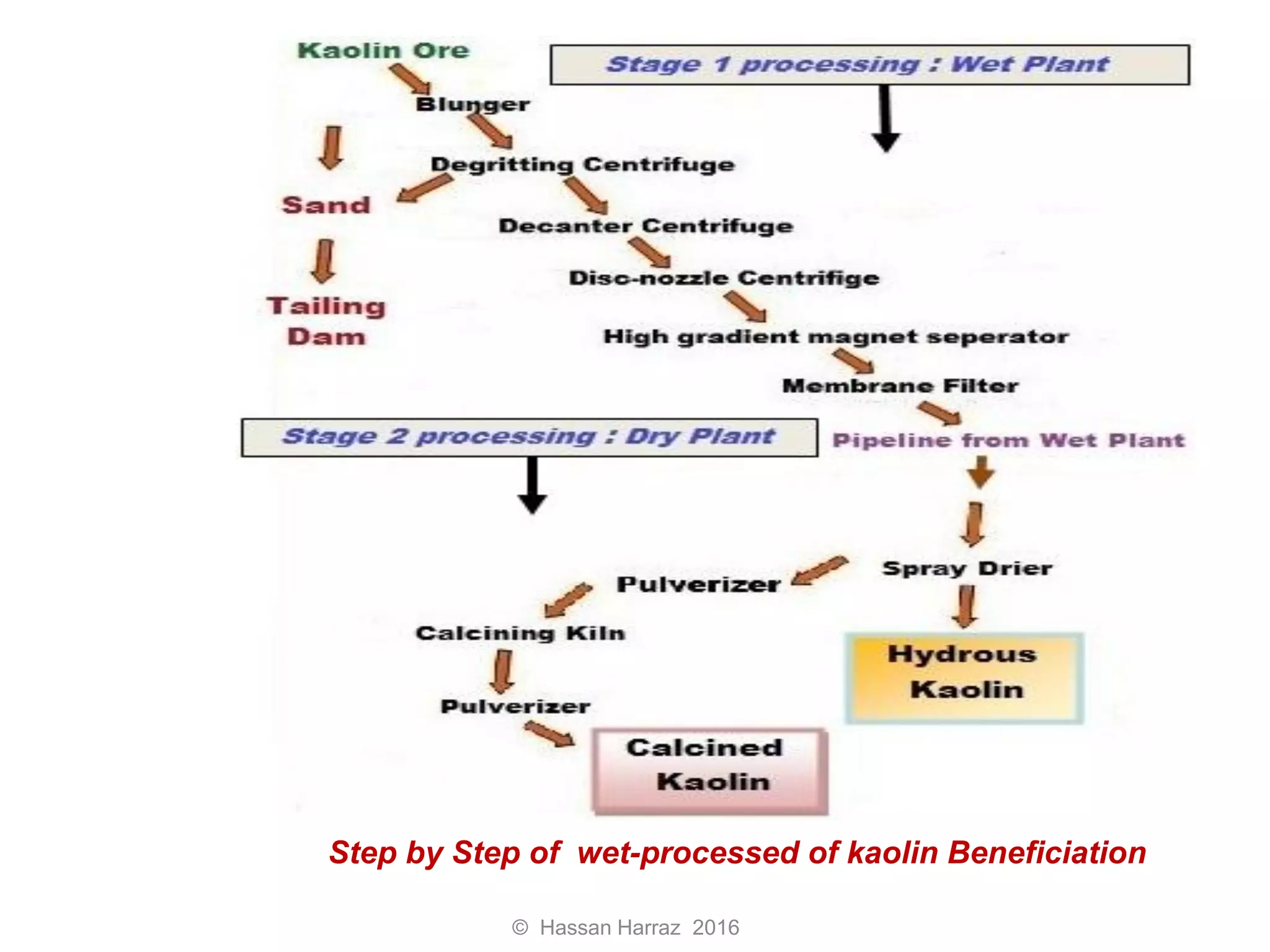
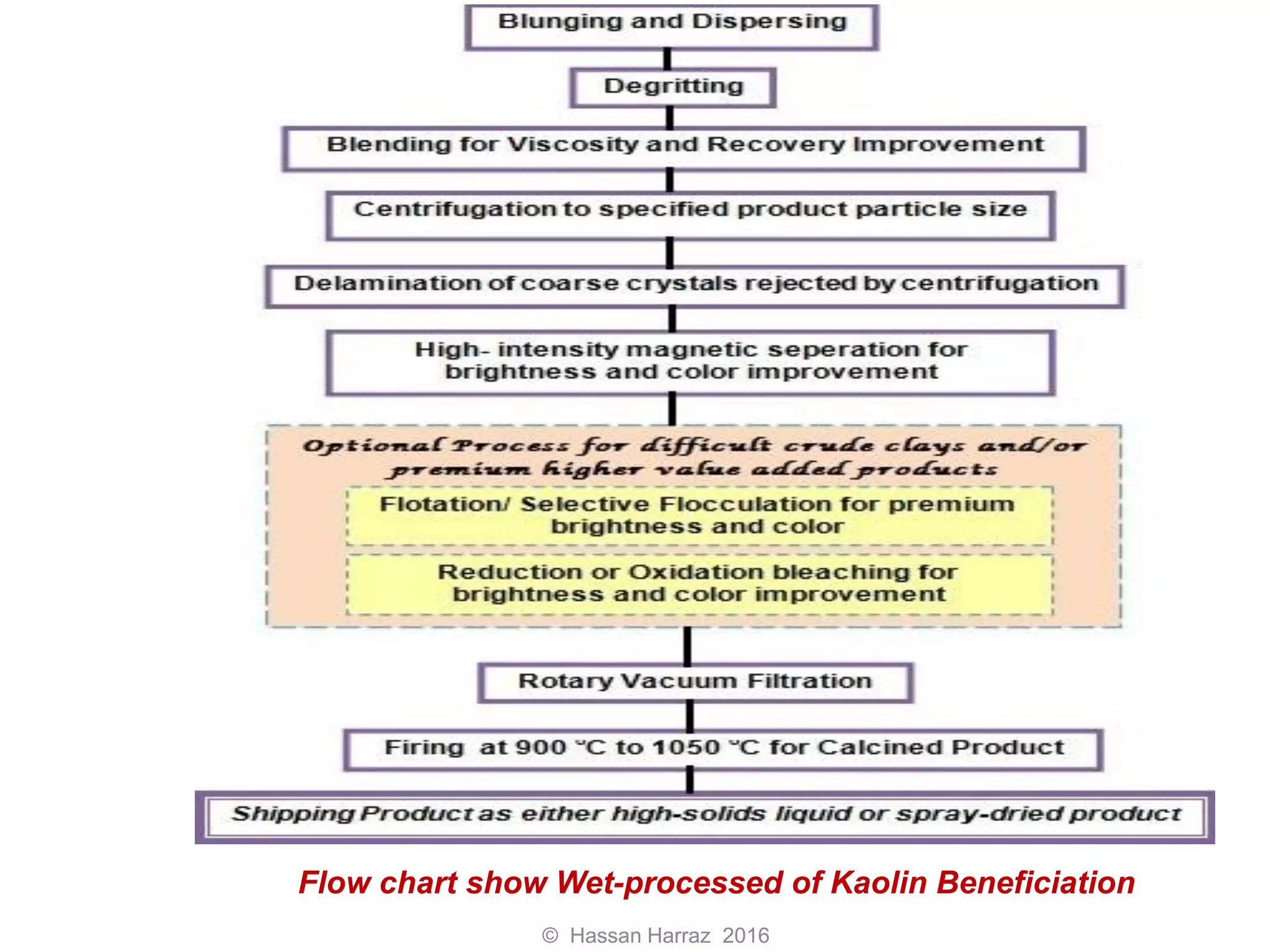
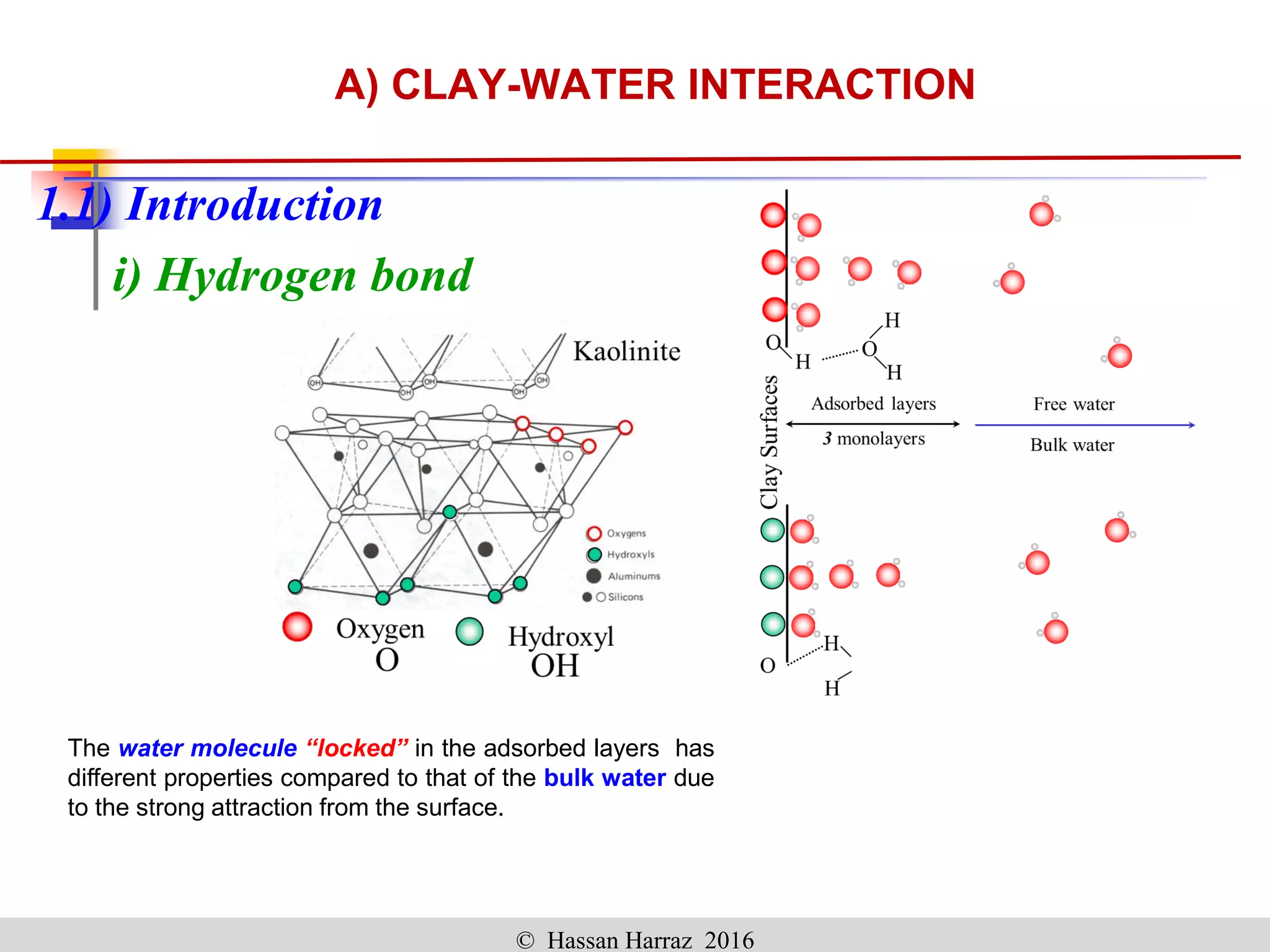
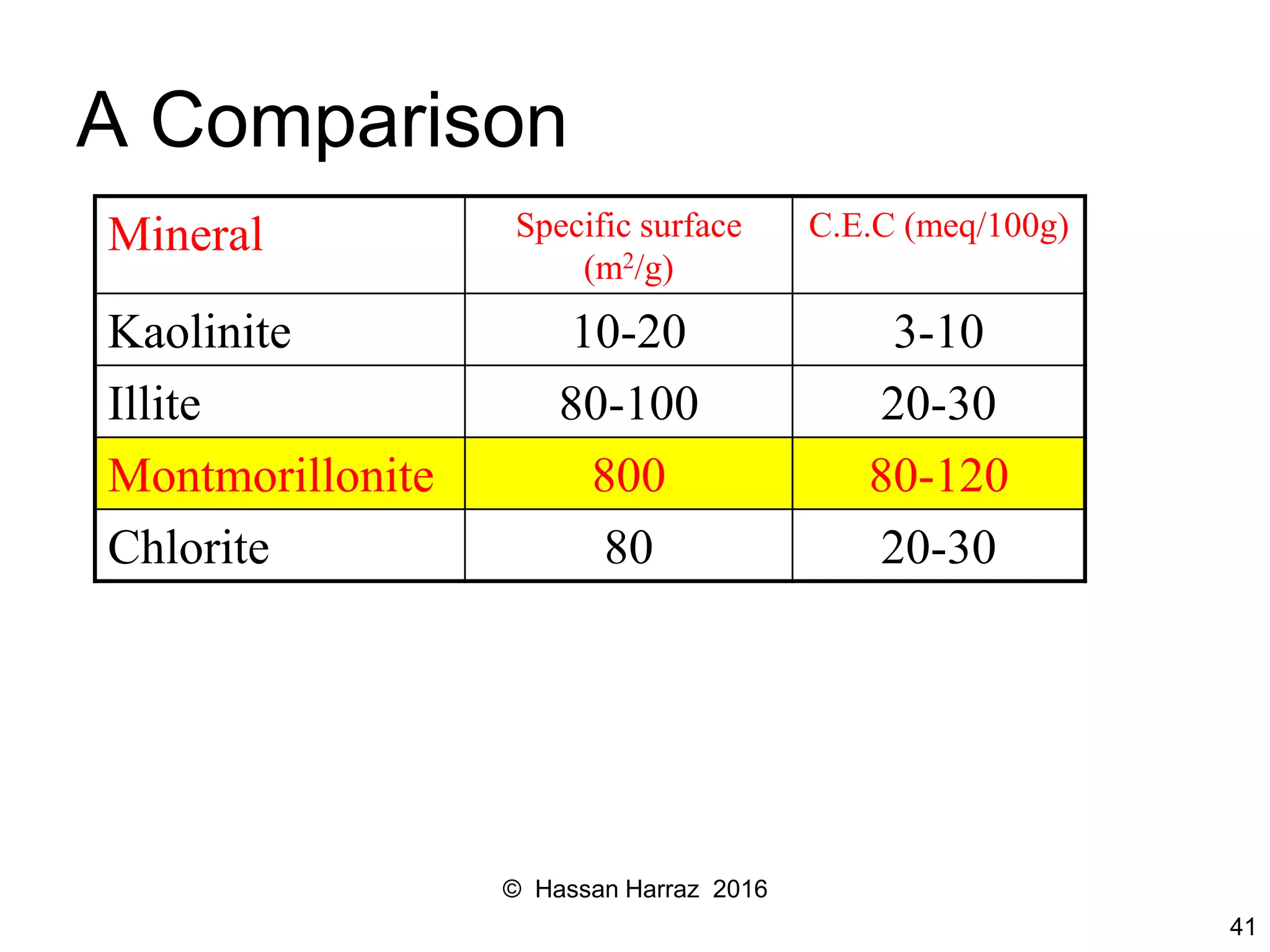
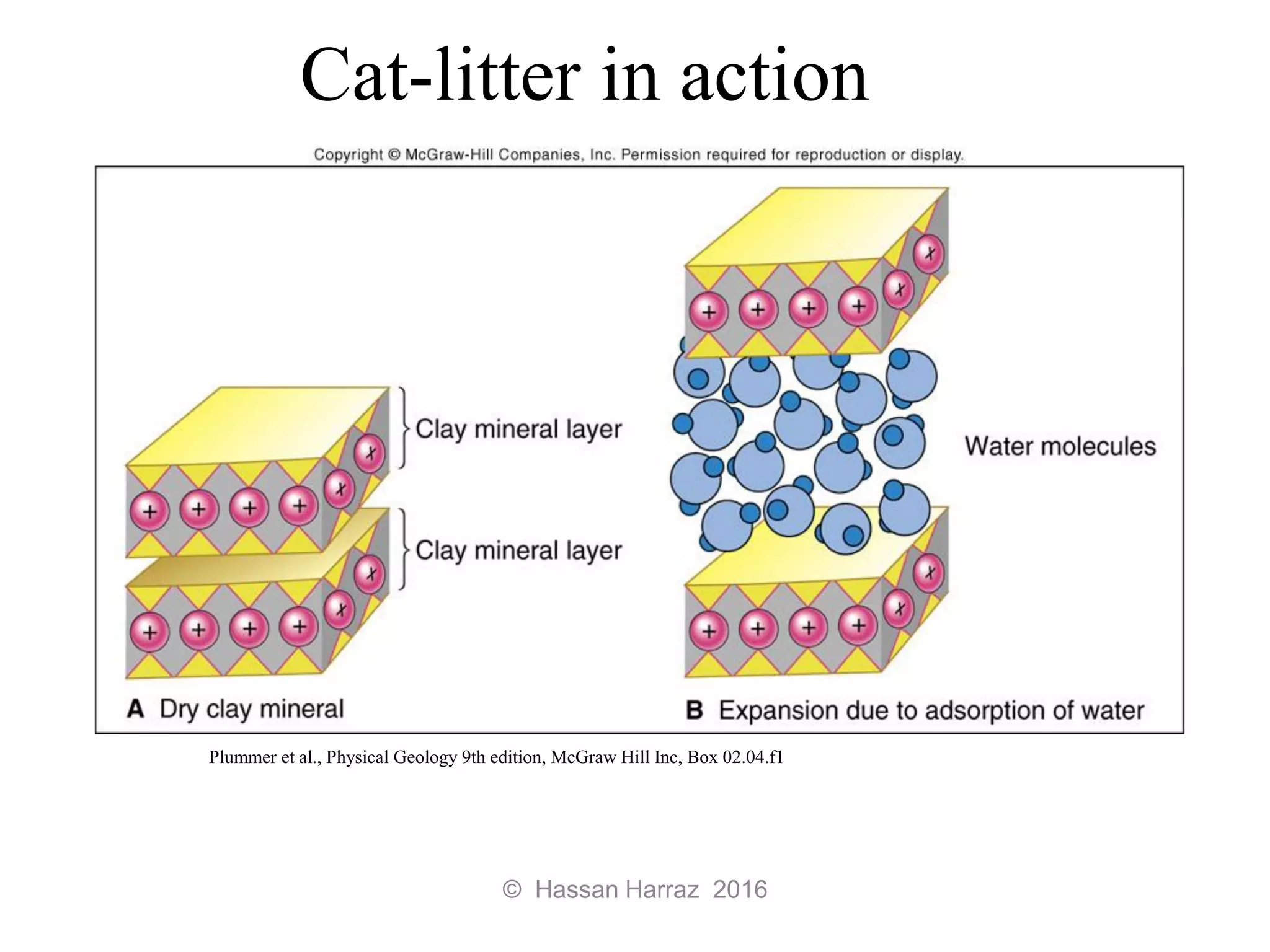
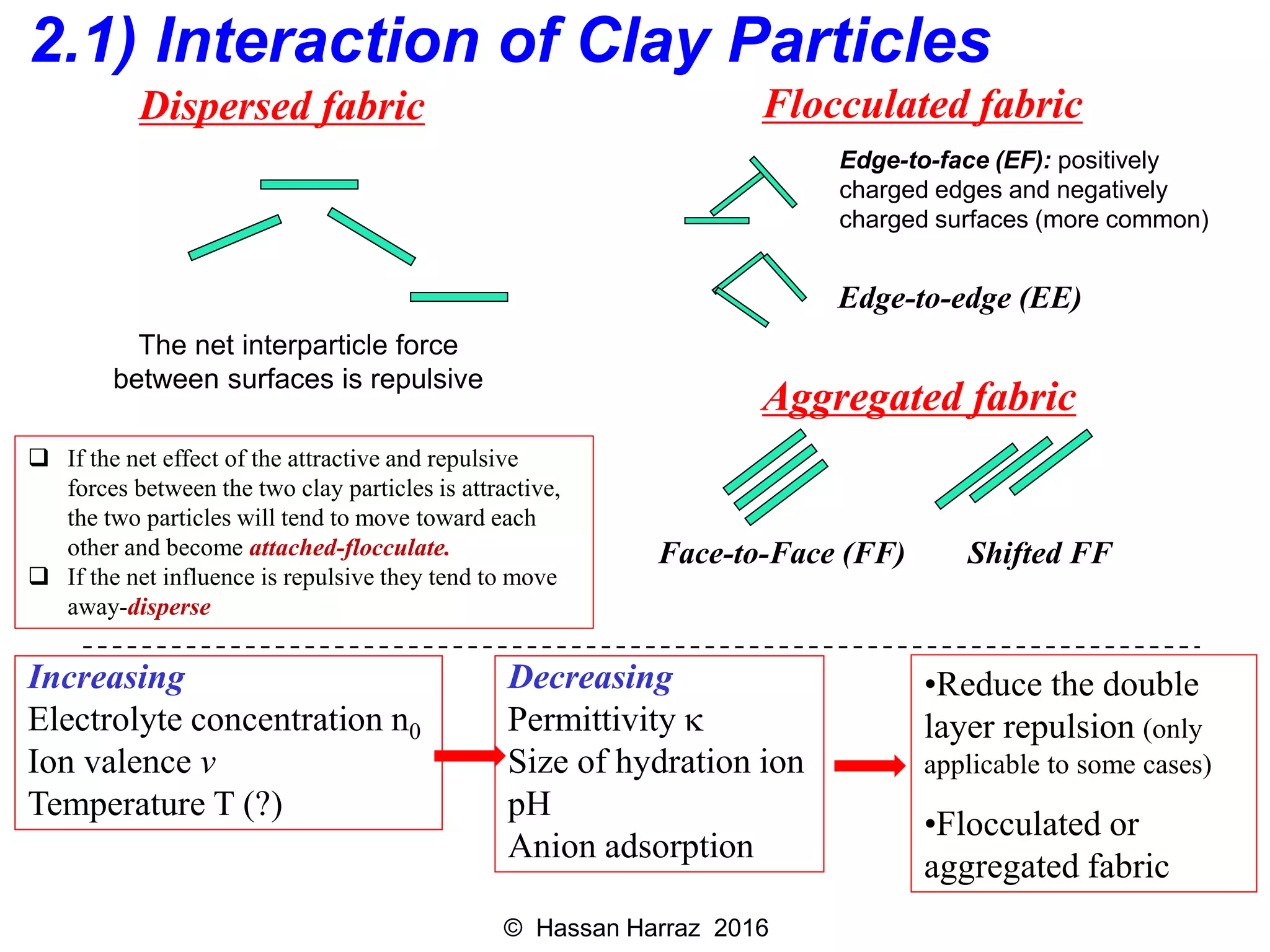
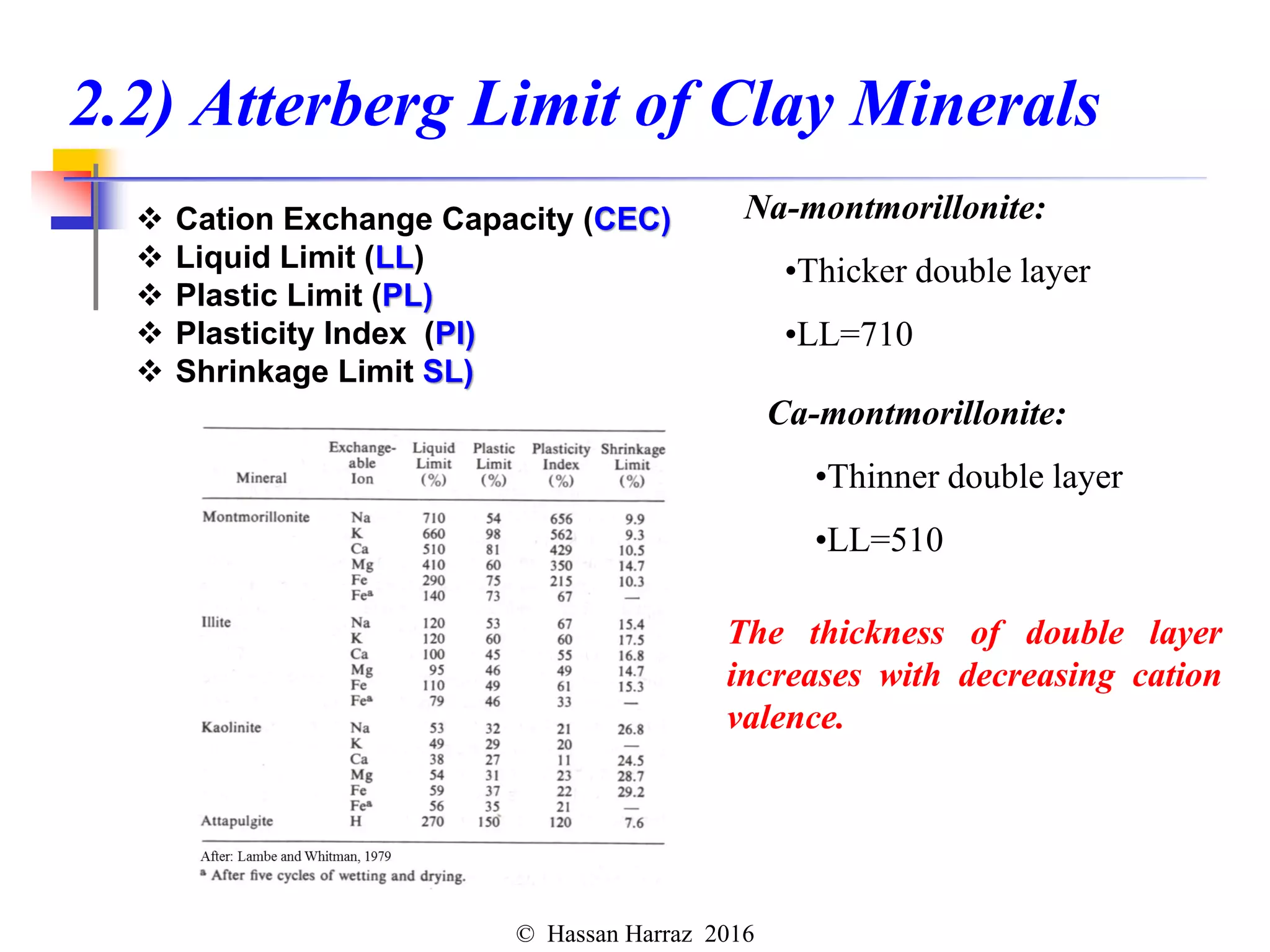
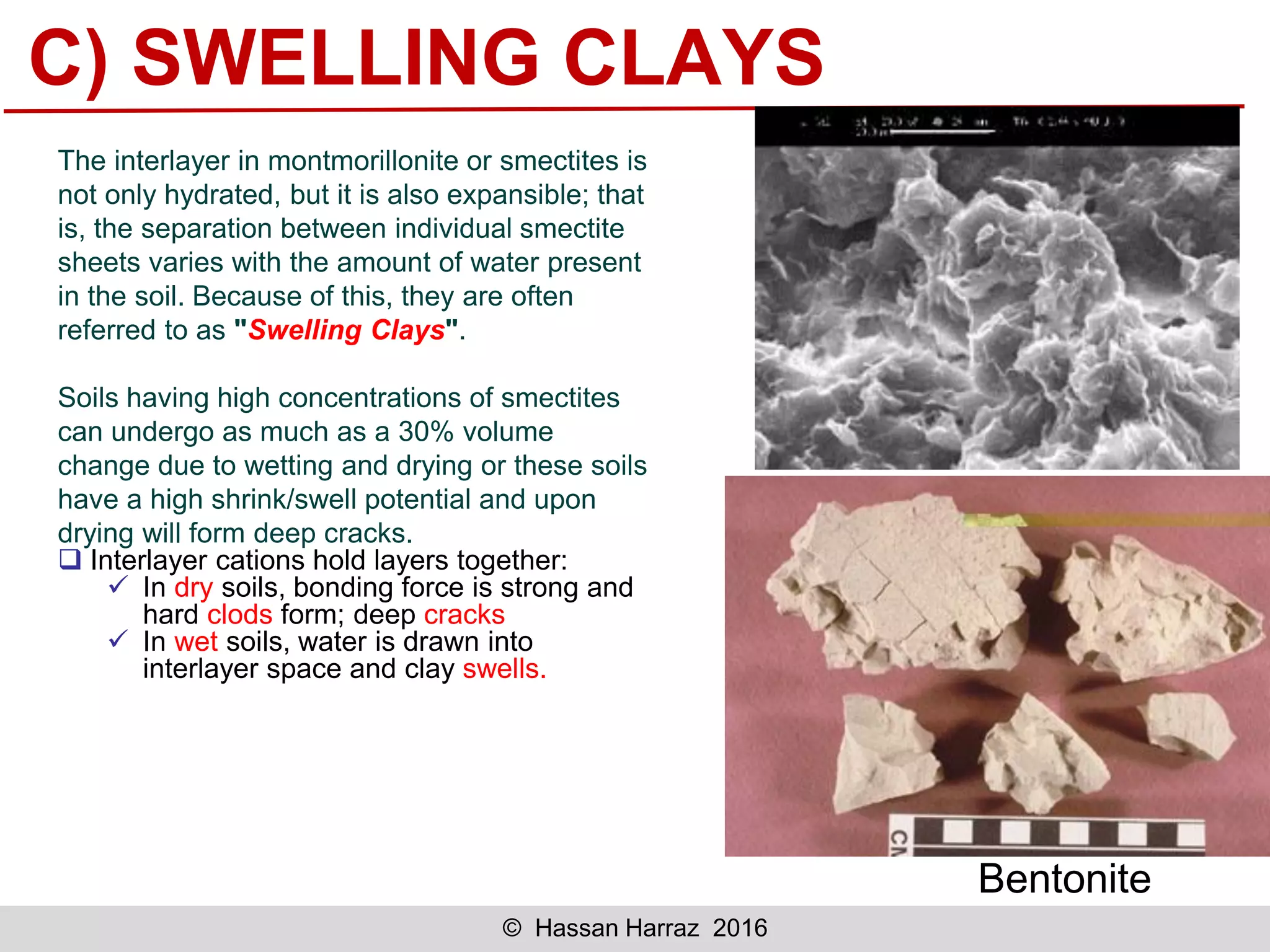
This document outlines the benefits and processing methods of clay minerals, highlighting their structures, types, and applications in various industries such as agriculture, ceramics, and construction. It details the properties of different clay types, including kaolin and montmorillonite, as well as the processes for their characterization and beneficiation. The interactions of clay minerals with water, their swelling properties, and the importance of ion exchange are also discussed.