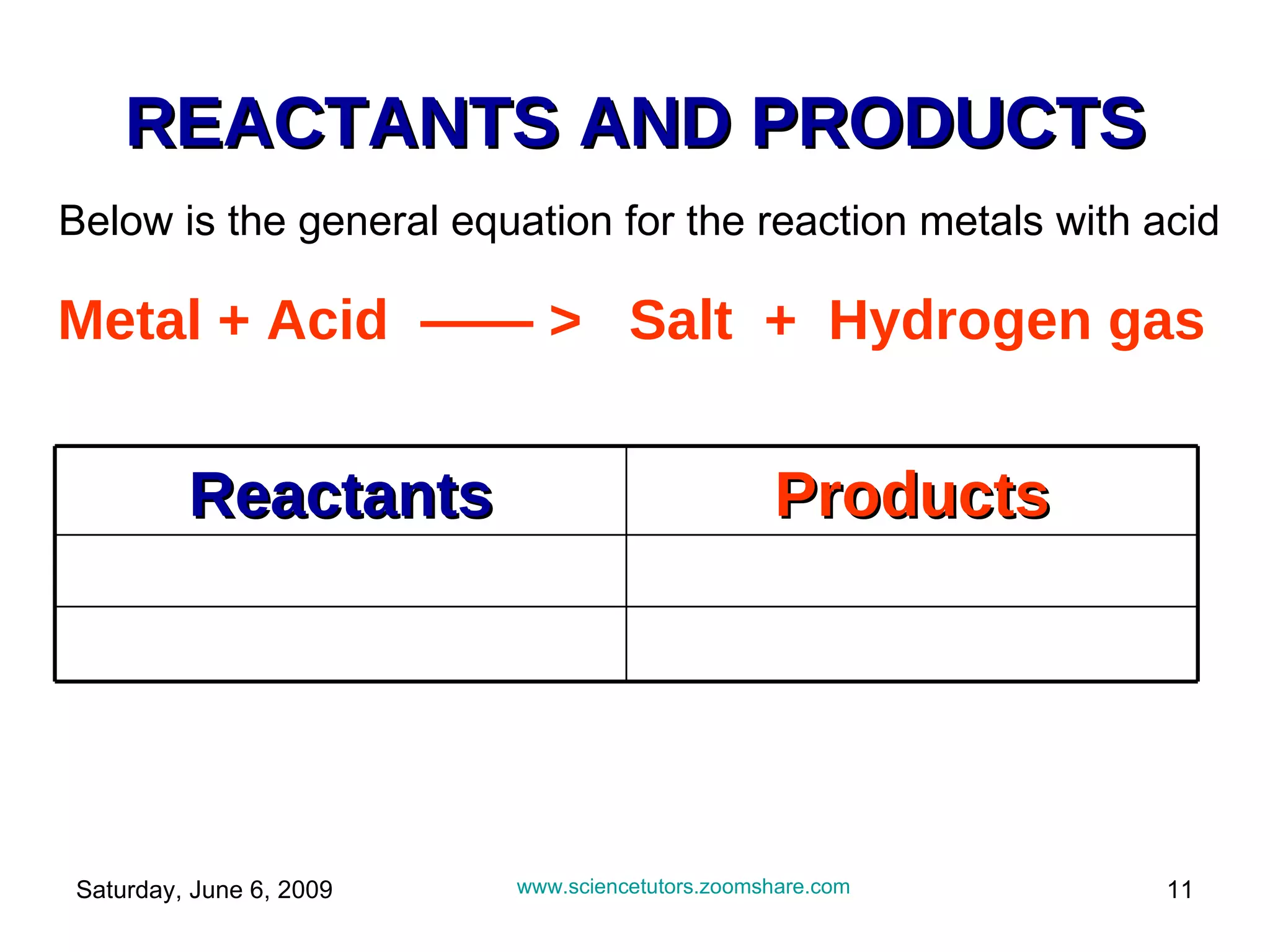
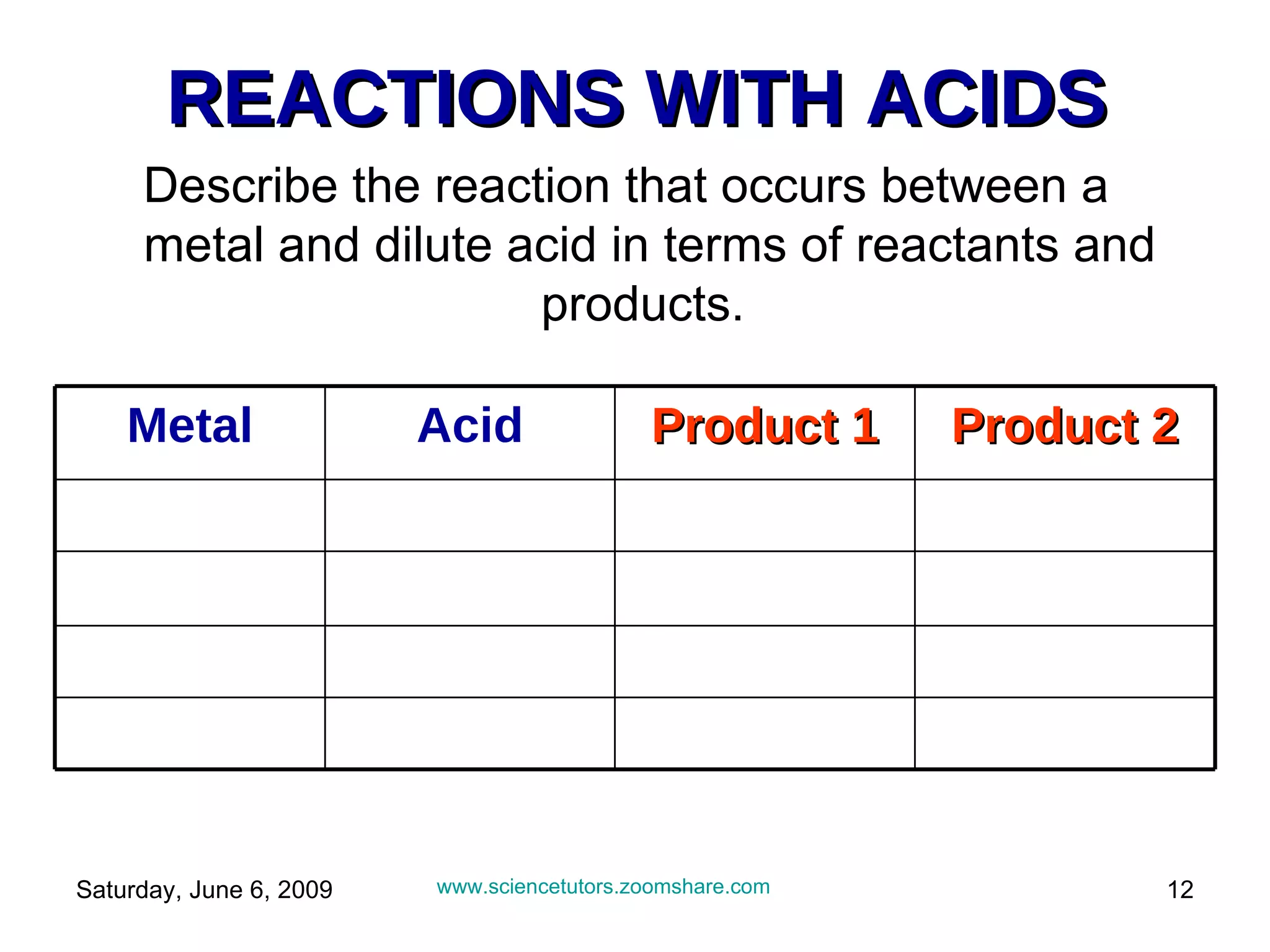
The document discusses the reactions of metals with acids and their uses. It explains that metals are found naturally in ores and can be extracted. Certain metals like aluminum, steel, and iron have specific uses like in aircraft, cooking pots, etc. due to their properties. It also describes how most metals react with acids to produce salts and hydrogen gas. The reactivity of metals follows certain patterns that allow predictions of how a metal will react with acid.