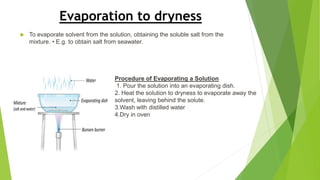
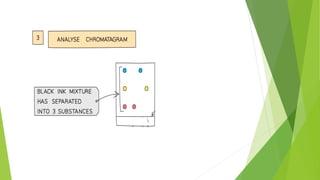
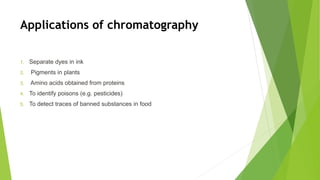
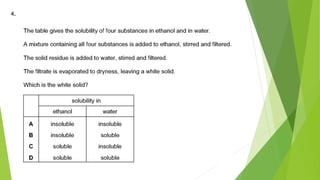
This document discusses various laboratory equipment and techniques used to measure elements, compounds, and mixtures. It describes apparatuses for measuring volume, time, mass, and temperature. Specific tools covered include beakers, measuring cylinders, burettes, pipettes, and gas syringes. It also discusses hazards in the laboratory and rules for working safely. Key concepts explained are the differences between elements, compounds, and mixtures, and techniques for checking purity and separating mixtures like chromatography, distillation, filtration, crystallization, and decantation.