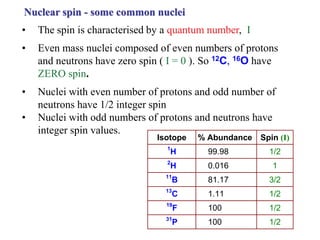
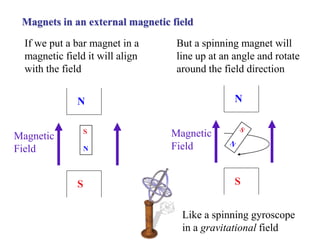
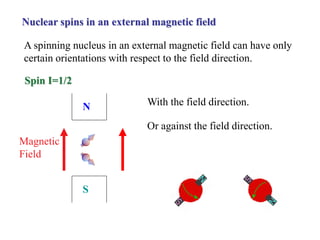
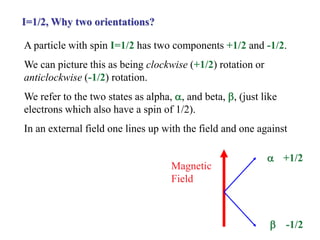
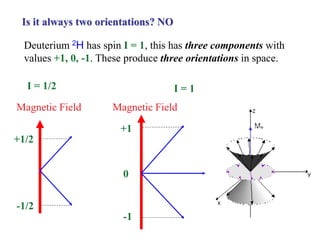
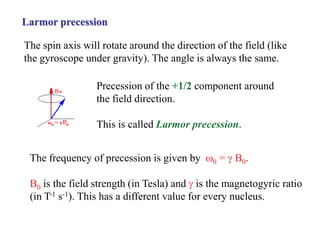
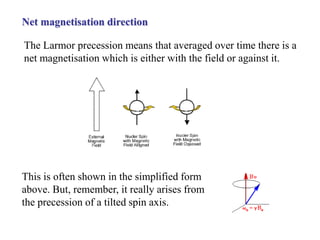
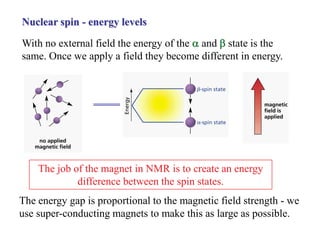
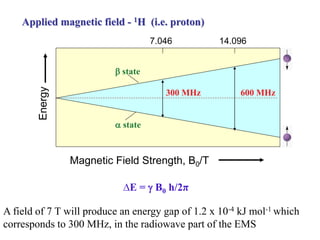
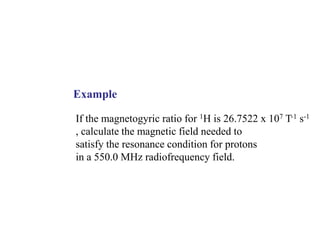
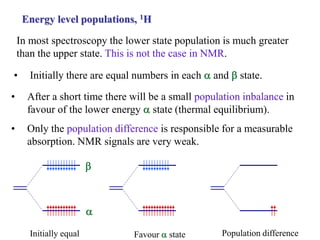
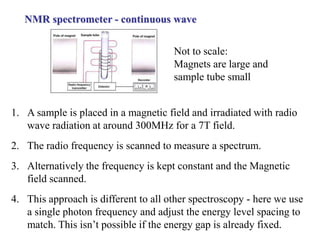
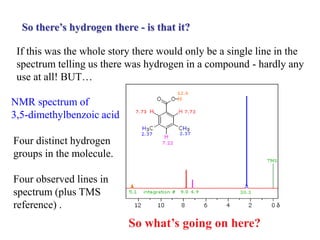
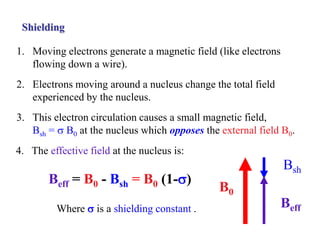
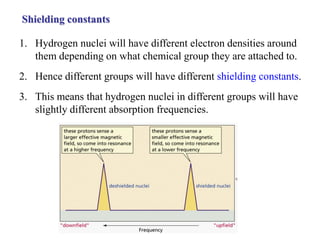
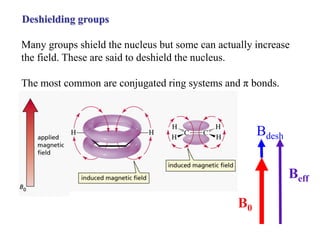
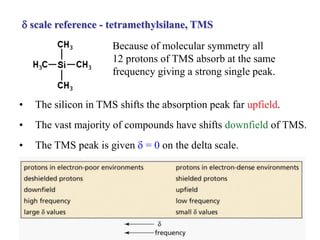
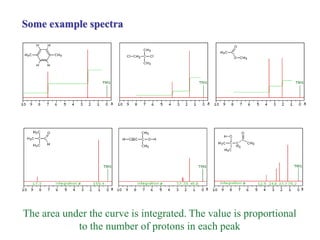
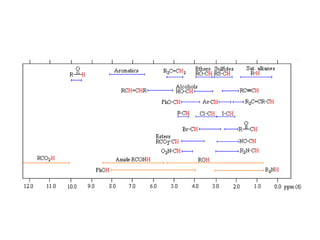
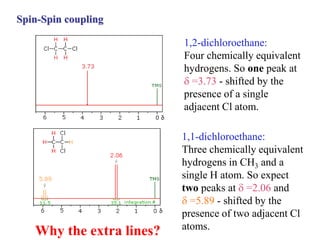
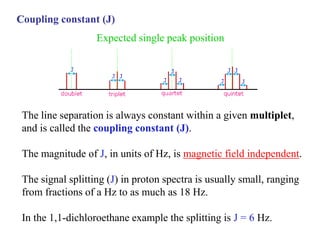
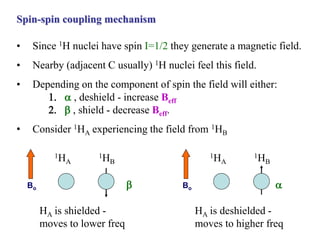
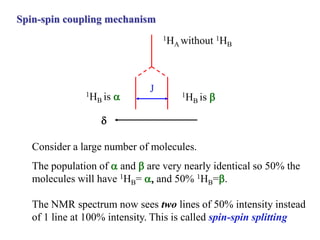
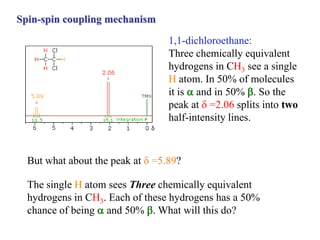
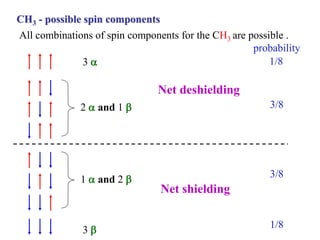
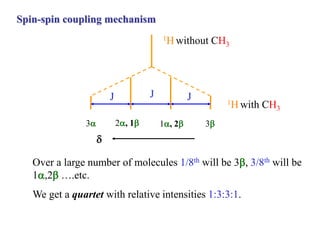
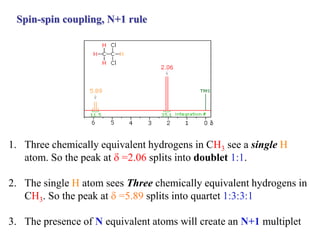
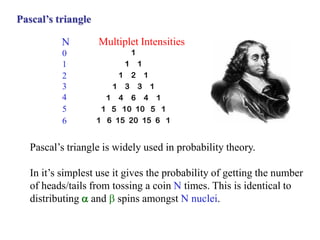
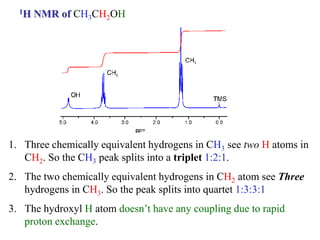
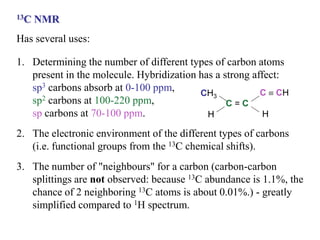
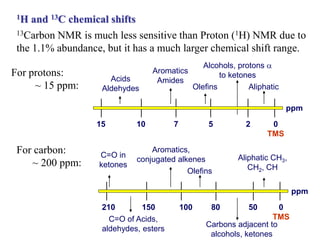
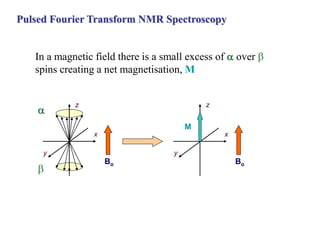
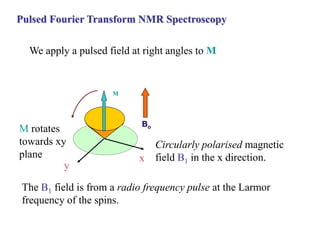
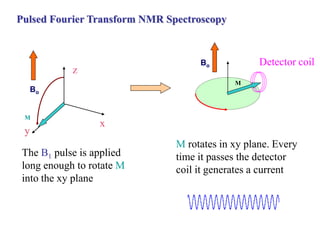
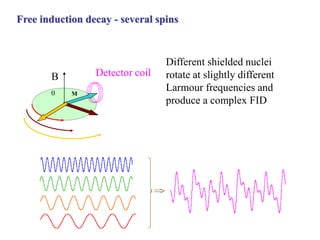
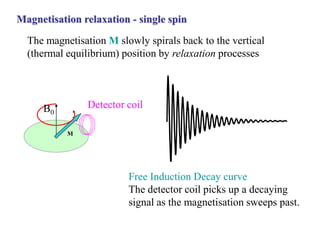
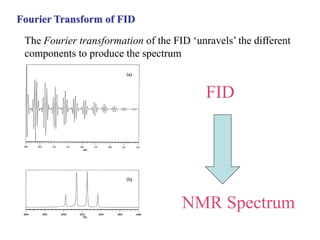
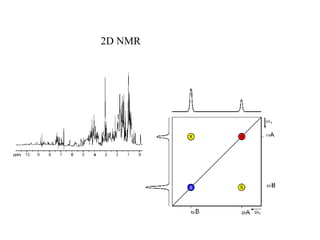
The document provides information on nuclear magnetic resonance (NMR) spectroscopy. It discusses key concepts such as nuclear spin, magnetic moments, energy levels of nuclei in magnetic fields, and spin-spin coupling. Nuclear spin gives rise to quantized magnetic moments and angular momentum. In an external magnetic field, these magnetic moments can align parallel or anti-parallel, splitting the energy levels. The energy difference between levels depends on the field strength and nucleus. NMR spectroscopy detects the absorption frequencies of nuclei as they transition between energy levels when irradiated with radio waves. Chemical shifts arise from electron shielding effects, allowing NMR to distinguish between similar nuclei. Spin-spin coupling further splits peaks into multiplets, providing detailed information about molecular structure.