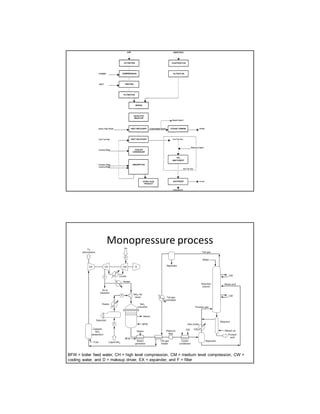
Nitric acid is predominantly used in the manufacture of fertilizers, explosives, and as an etching agent for metals. Its production involves various processes, notably the Ostwald process, which efficiently converts ammonia and air into nitric oxide, subsequently oxidized to form nitric acid. Modern production methods focus on reducing nitrogen oxide emissions and improving energy efficiency through monopressure and dual-pressure processes.