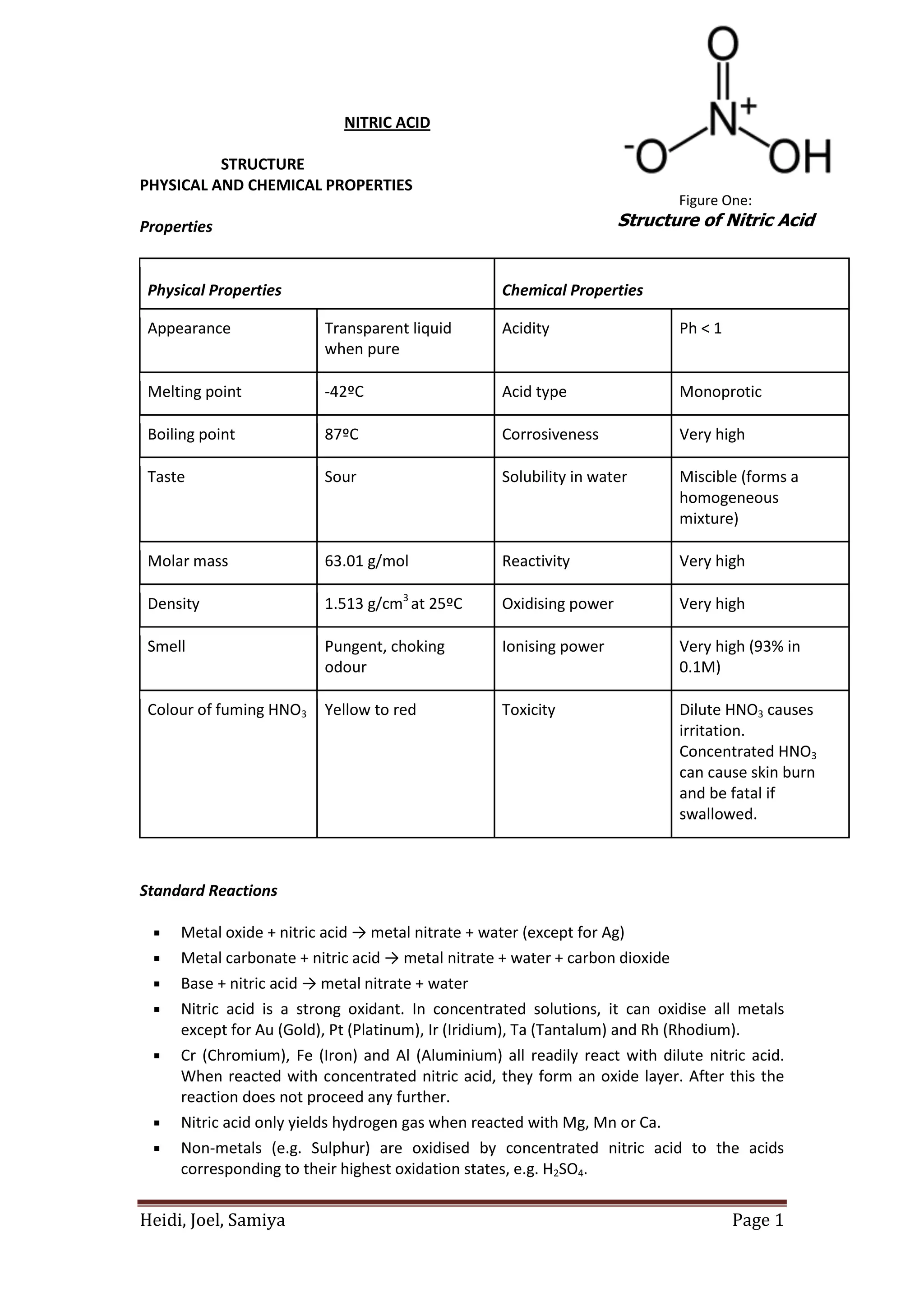
Nitric acid is a strong, highly corrosive acid that is colorless when pure. It is miscible in water and has a high density. Nitric acid is produced industrially through the oxidation of ammonia or through electric arc processes. It is used to produce fertilizers, explosives, and other nitrogen-containing compounds. Nitric acid poses environmental risks such as acid rain and water contamination if released.
![Nitric acid -_summary_sheet[1][1]](https://image.slidesharecdn.com/nitricacid-summarysheet11-101010215844-phpapp01/85/Nitric-acid-_summary_sheet-1-1-2-320.jpg)
![Nitric acid -_summary_sheet[1][1]](https://image.slidesharecdn.com/nitricacid-summarysheet11-101010215844-phpapp01/85/Nitric-acid-_summary_sheet-1-1-3-320.jpg)