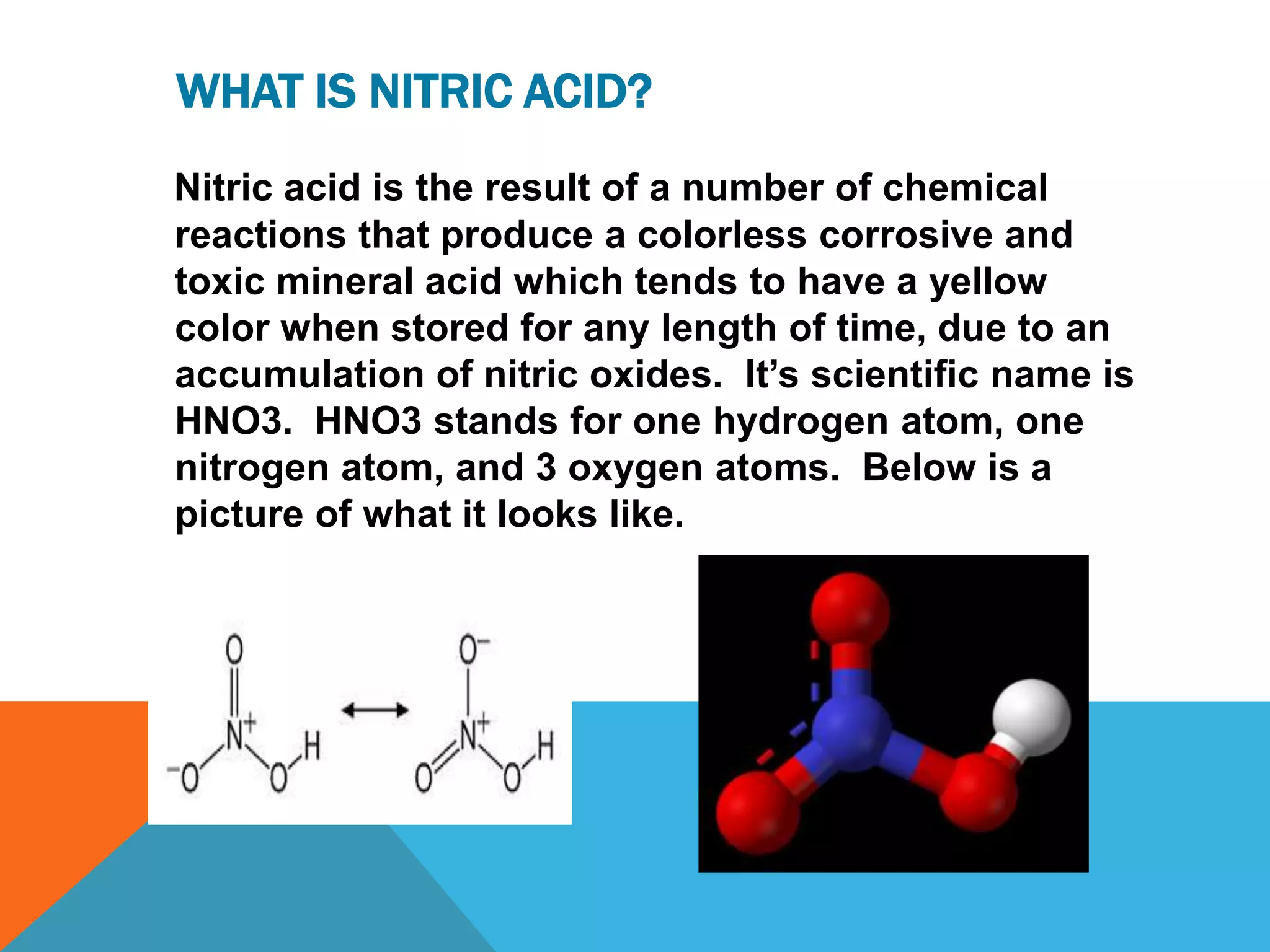
Nitric acid is a colorless, corrosive, and toxic mineral acid. It is composed of one hydrogen atom, one nitrogen atom, and three oxygen atoms. Nitric acid was first identified in the 9th century and has had various names over time. It was first synthesized in the early 20th century and was used by Germans prior to WWI to produce nitrates for weapons. Nitric acid is commonly used in industry, agriculture, and for making explosives and rocket fuel due to its strong oxidizing properties. It poses severe health and safety hazards if mishandled.