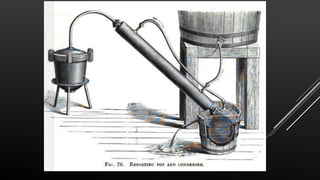
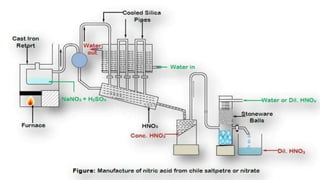
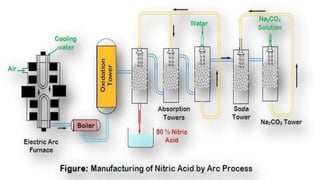
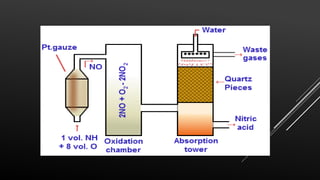
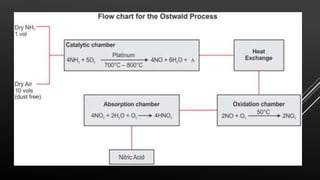
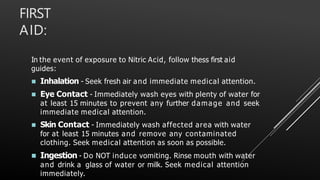
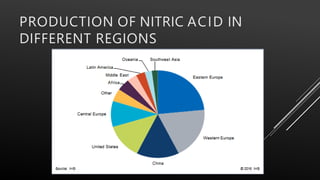
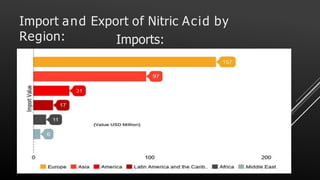
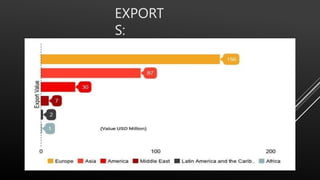
This document provides information about nitric acid, including its physical and chemical properties, uses, manufacturing process, and production in Pakistan. It describes nitric acid as a colorless liquid used to make fertilizers, dyes, and explosives. Three common industrial methods for producing nitric acid are outlined - the Chile saltpeter method using sodium nitrate, Birkeland-Eyde's method using air, and Ostwald's method using ammonia. Safety precautions for handling nitric acid and first aid measures for exposure are also summarized.