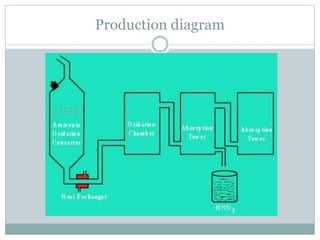
The document details the manufacturing process of nitric acid through the Ostwald process, which involves converting ammonia into nitric oxide and then into nitrogen dioxide before absorption in water to form nitric acid. Key steps include the oxidation of ammonia, secondary oxidation to produce nitrogen dioxide, absorption of nitrogen dioxide to form dilute nitric acid, and concentration of the acid using concentrated sulfuric acid. Nitric acid is essential in various applications, including fertilizers, explosives, and pharmaceuticals.